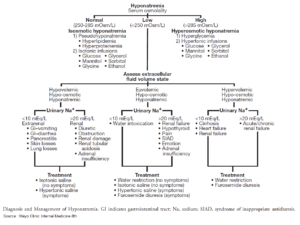
Definition, Diagnosis, and Clinical Features
The serum calcium concentration has a major impact on membrane excitability, especially in the heart, and therefore the serum Ca concentration is tightly regulated. Hypercalcemia is defined as an increase of serum calcium to 2.6 mmol/L, hypocalcemia as a decrease to 2.1 mmol/L.
Diagnosis
About 50% of serum calcium is protein bound. For this reason, the total serum calcium concentration can only be interpreted with knowledge of the serum albumin concentration. The concentration of ionized free biologically active serum Ca is about 1.2 mmol/L. In the context of hypoalbuminemia, ionized serum calcium concentration can be obtained as follows:
- direct measurement of ionized (free) Ca by blood gas analysis
- mathematical correction based on the serum albumin concentration with the following formula:Δ [Ca] (mmol/L) = 0.02 × Δ [albumin] (g/L)
Clinical Features
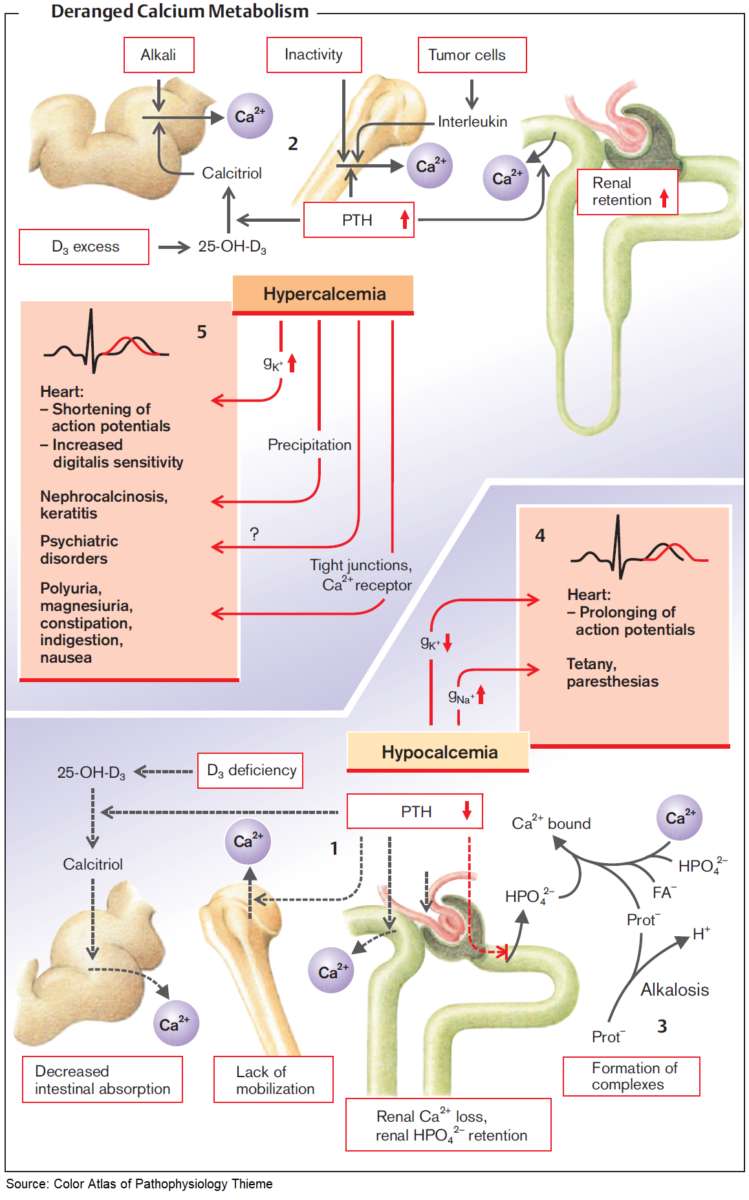
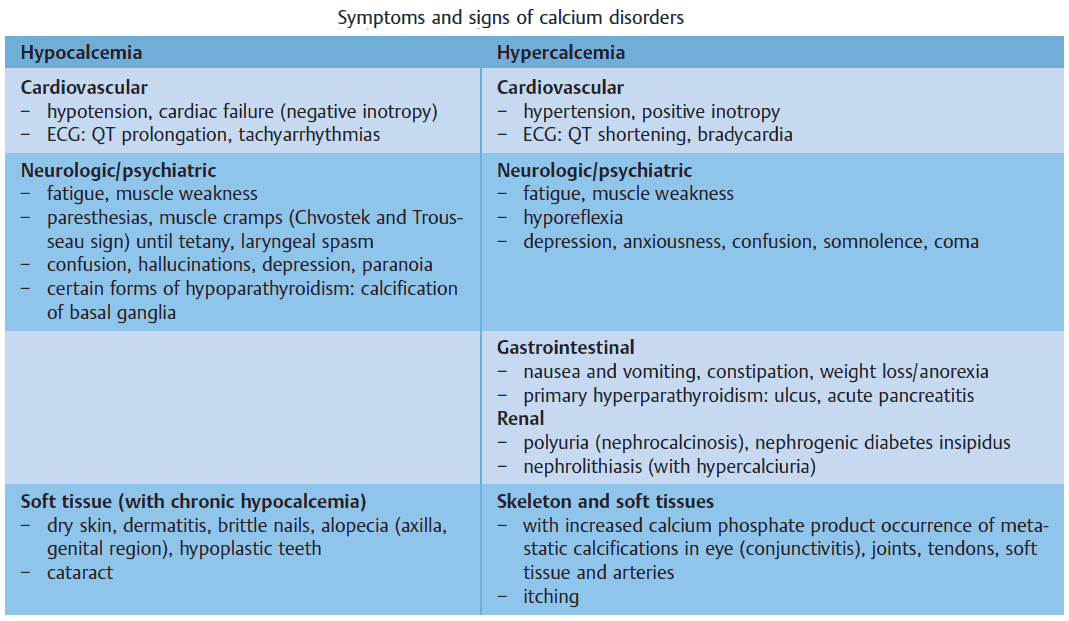
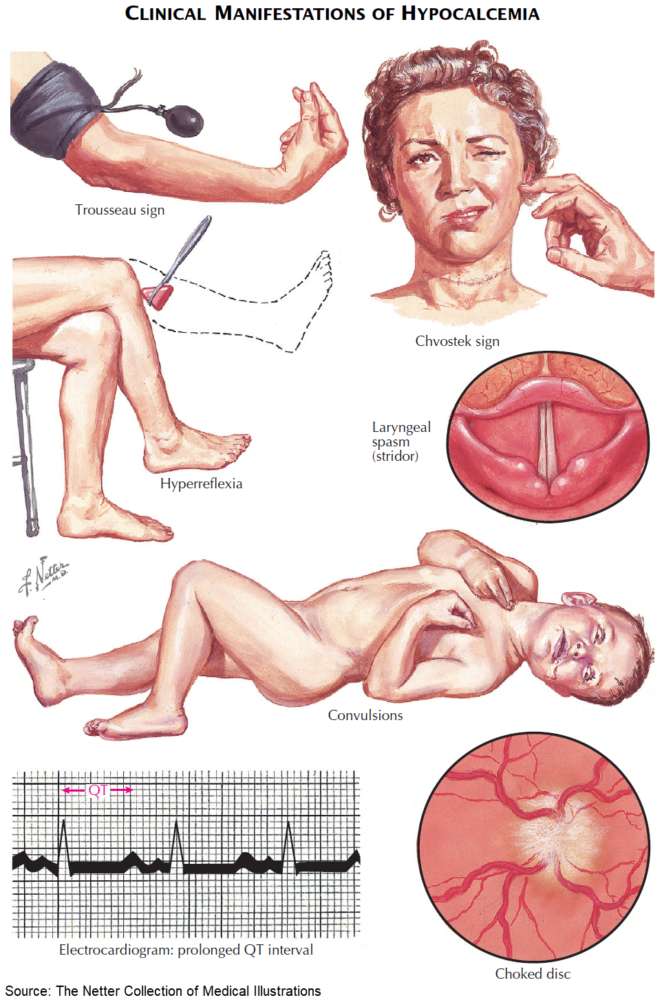
The clinical symptoms and signs of calcium disorders are mainly an expression of disturbed membrane excitability. A systematic overview is shown in the image below. In general, hypocalcemia leads to enhanced, and hypercalcemia to reduced membrane excitability.
Ca and PO4 3– can form complexes in serum. When the calcium-phosphate product ([Ca] × [PO4 3–]) rises above 4.8 (mmol/L)² in serum, this can result in metastatic calcification of organs and tissues (nephrocalcinosis; pulmonary calcification; calciphylaxis: metastatic calcifications in vessels, tendons, joints, and fatty tissue).
Causes
Calcium disorders can arise via four different pathophysiologic mechanisms:
- disorders of parathyroid function and regulation
- disorders of vitamin D metabolism and intestinal absorption
- disorders of bone metabolism
- disorders of renal calcium excretion.
Hypocalcemia (PCa < 2.1 mmol/L)
Causes of Hypocalcemia
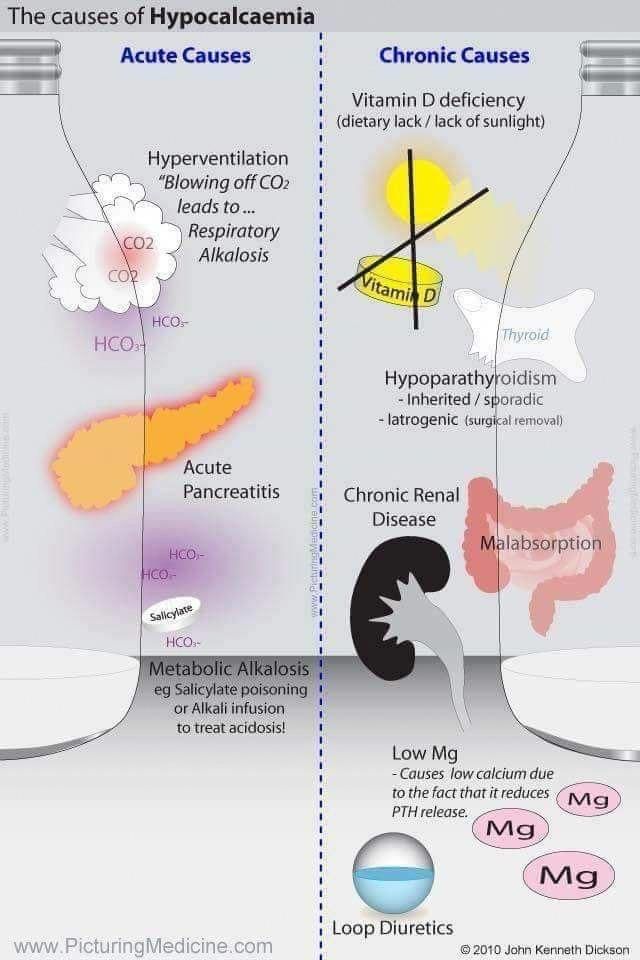
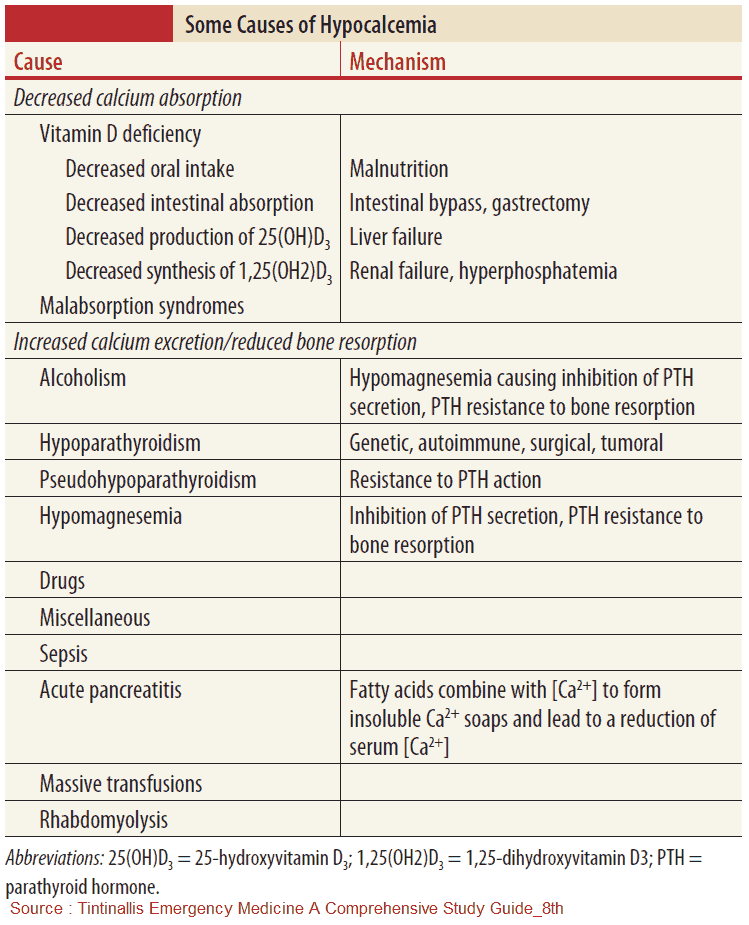
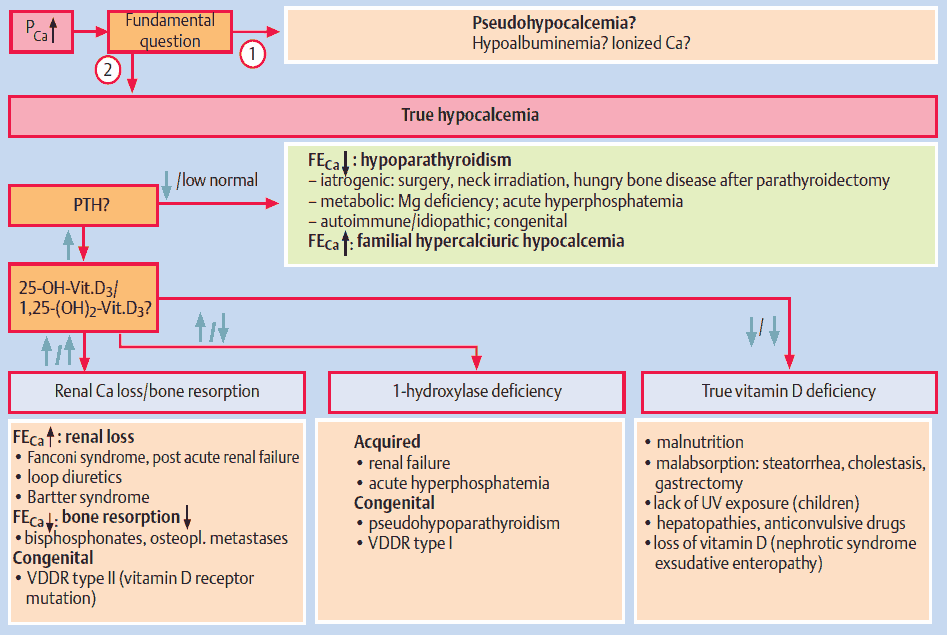
Differential Diagnosis of Hypocalcemia
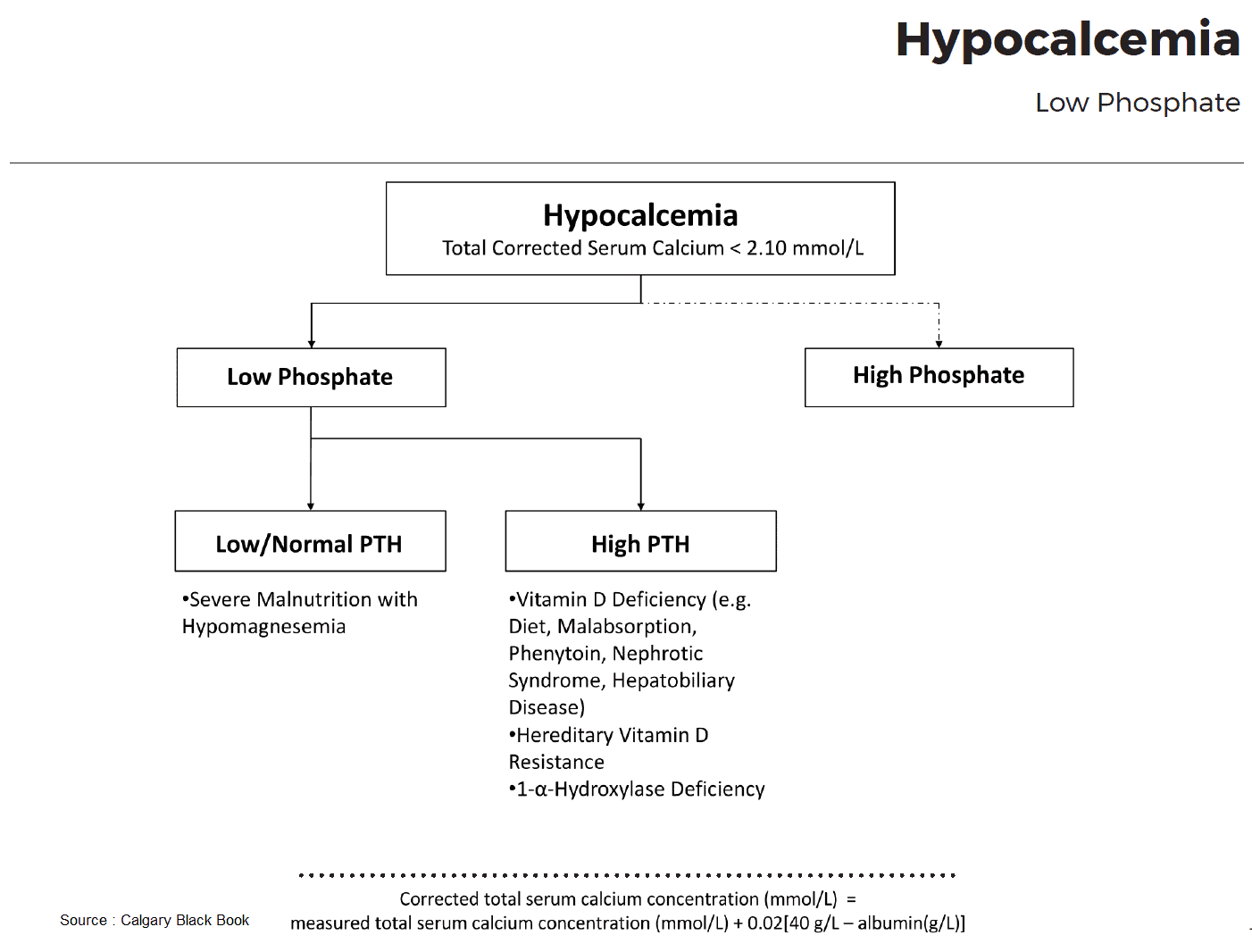
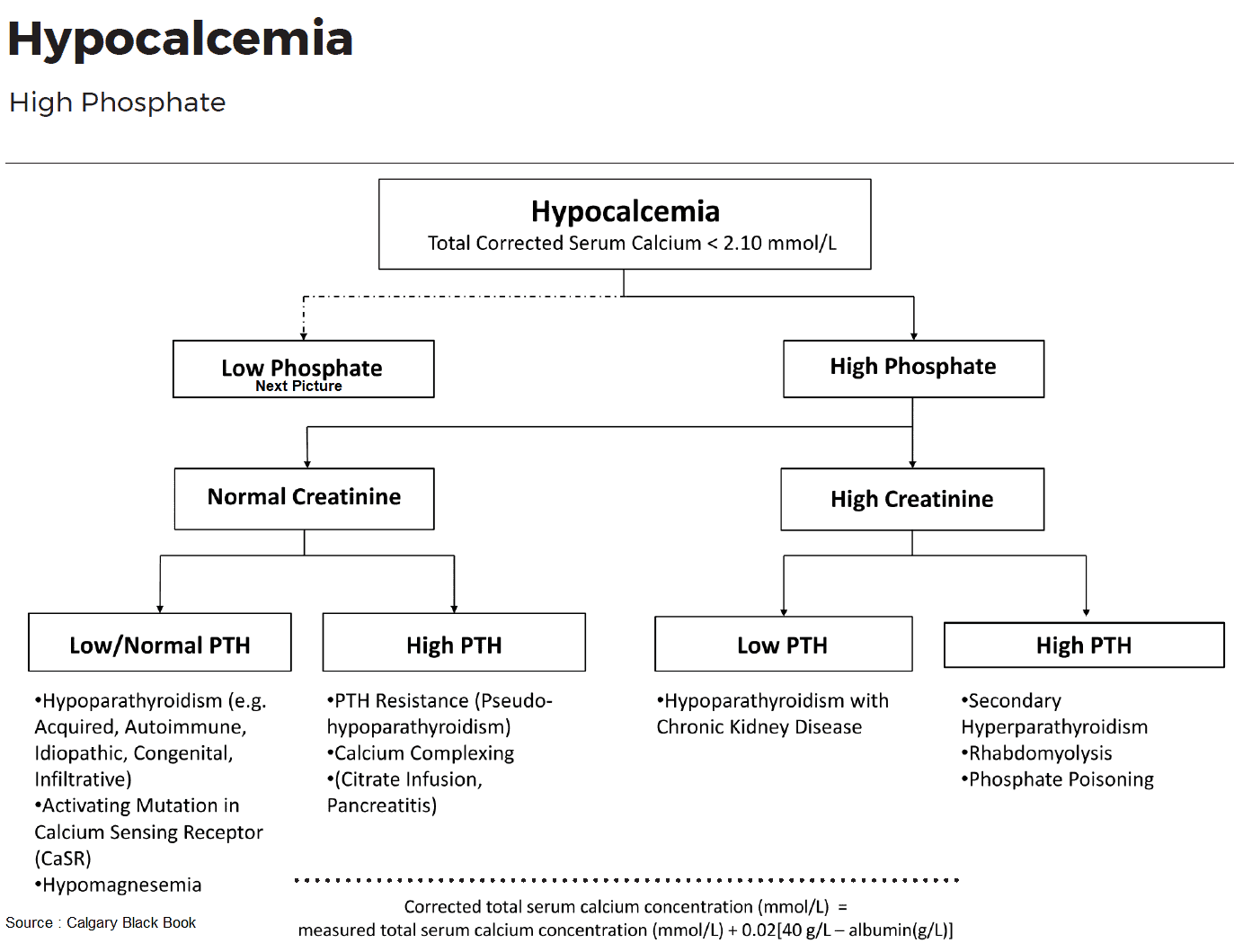

Hypoparathyroid Status
True hypoparathyroidism with low PTH values has to be distinguished from pseudohypoparathyroidism with PTH resistance. The latter is rare and occurs in the context of congenital disorders, e. g., Albright osteodystrophy. Also rare, but pathophysiologically very interesting, is hypoparathyroidism due to an activating mutation of the “calcium-sensing receptor” (CSR), which imitates a hypercalcemia signal and therefore leads to inhibition of PTH secretion. The disease is called familial hypercalciuric hypocalcemia.
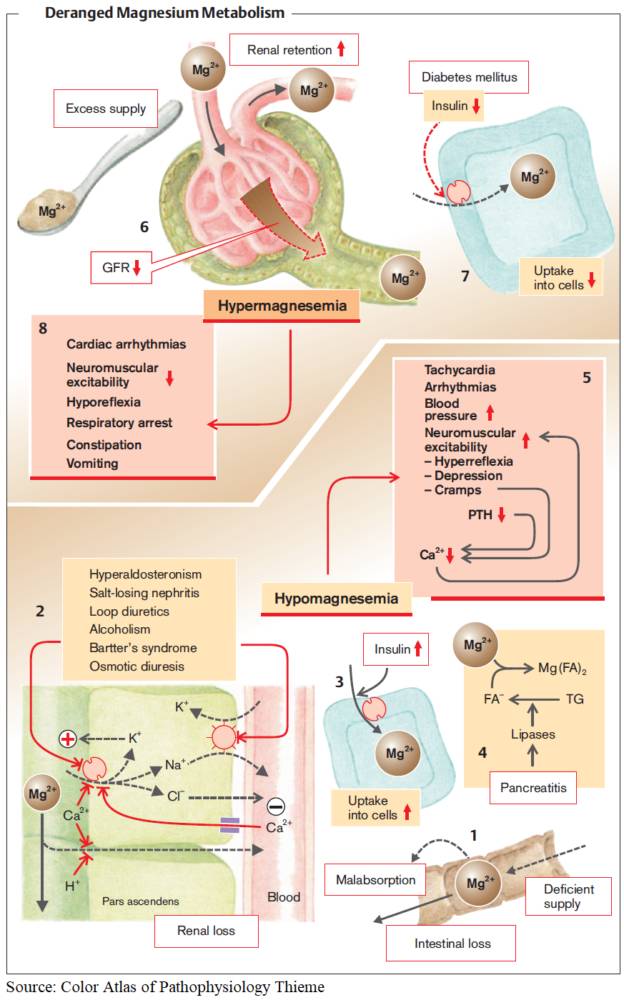
The most frequent causes are disorders of the parathyroid gland itself. PTH deficiency can occur following surgical interventions (thyroidectomy, parathyroidectomy) or after irradiation of the neck area. Some metabolic disorders (e. g., hyperphosphatemia and hypomagnesemia) can also lead to hypoparathyroidism with hypocalcemia.
Hypomagnesemia leads to impaired PTH secretion and PTH resistance and together with hypocalcemia to tetany. Finally, several autoimmune and infiltrative diseases can lead to destruction of the parathyroid glands.
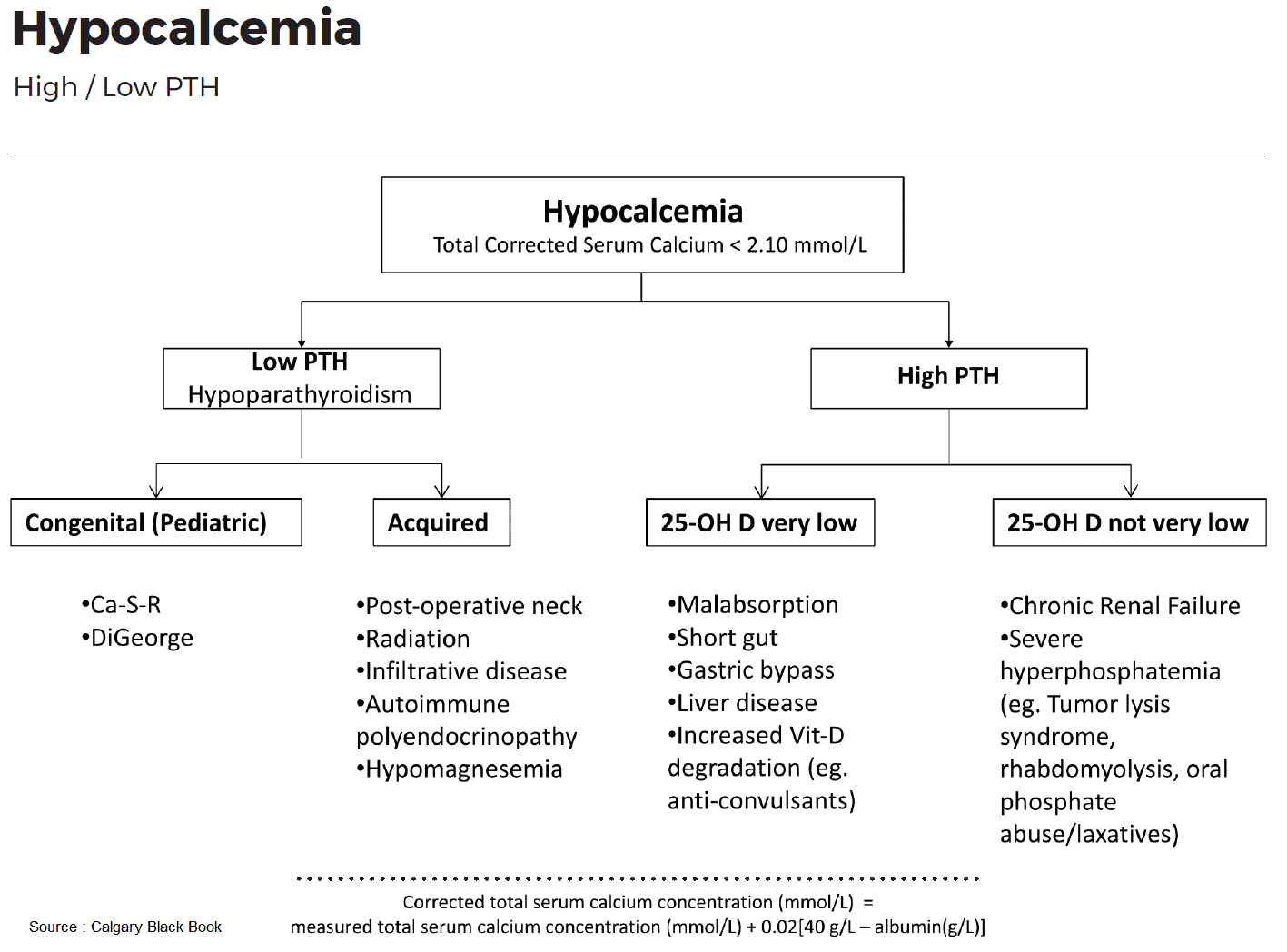
Vitamin D Deficiency
Severe malnutrition and/or malabsorption reduces the intake of vitamin D3 as well as calcium, whereas a severe nephrotic syndrome leads to loss of vitamin D and its serum binding protein (rickets with vitamin D deficiency).
The image above lists the causes of inefficient or incomplete activation of vitamin D at three different levels. Two frequent causes are chronic renal insufficiency and hyperphosphatemia. In both cases 1-hydroxylation of vitamin D3 is impaired. Furthermore, hyperphosphatemia leads to hypocalcemia, which results in the classical constellation of secondary hyperparathyroidism with hyperphosphatemia and hypocalcemia.
Rare congenital diseases of vitamin D metabolism are caused either by a mutation of 1-hydroxylase (vitamin D-dependent rickets, VDDR type 1) or by a mutation of the vitamin D receptor (target organ resistance, VDDR type 2).
The consequences of vitamin D deficiency are reduced intestinal absorption of Ca resulting in hypocalcemia and reduced bone mineralization with typical signs of rickets in children and osteomalacia in adults.
Calcium Sequestration in Bones and Tissues
The state in which calcium deposition in the bones is massively increased is called “hungry bone disease”. It occurs with vitamin D therapy of severe rickets, but also after parathyroidectomy due to hyperparathyroidism, when empty calcium stores in the bones are replenished.
An overdose of bisphosphonates or severe osteoplastic metastases can also lead to hypocalcemia due to deposition in the bones.
Sequestration of calcium is observed in the context of acute pancreatitis (in soft tissues) and of transfusions with citrate-rich blood products (in the blood). The pathogenesis of hypocalcemia associated with sepsis and acute respiratory alkalosis (hyperventilation) is unclear.
Renal Calcium Loss
The most frequent cause of renal tubular calcium loss is medication with loop diuretics. In this case it is often associated with hypokalemia, hypomagnesemia, and alkalosis. However, all nephropathies with a proximal tubular damage (Fanconi syndrome) can lead to renal calcium loss. Finally, respiratory or metabolic acidosis leads to calcium loss in the kidney.
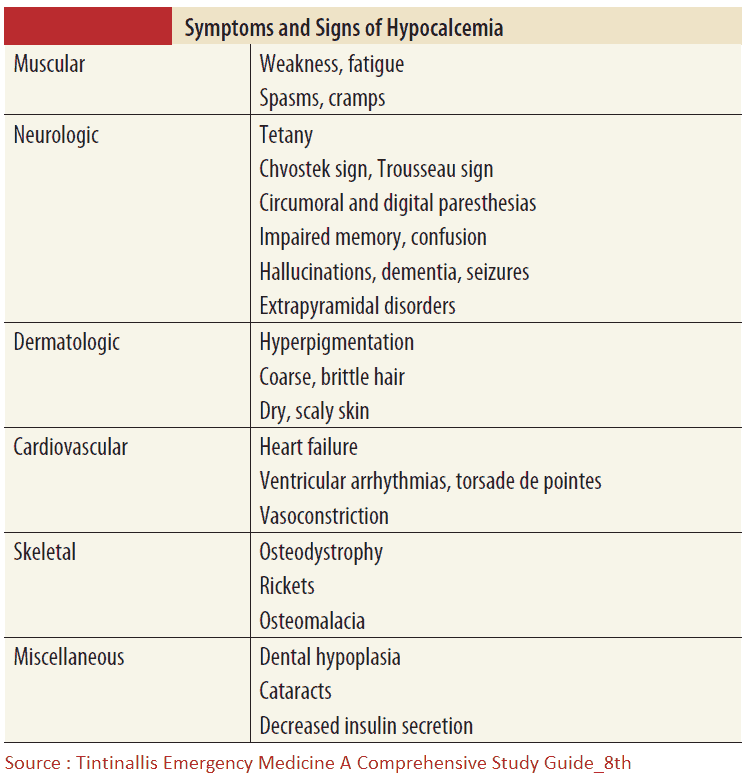
Symptoms and Signs of Hypocalcemia
Treatment of Hypocalcemia
- Acute / symptomatic (e.g., tetany, seizures, arrhythmias):
- IV calcium gluconate (preferred) or calcium chloride (central line)
- Continuous cardiac monitoring during infusion
- Chronic / mild cases:
- Oral calcium supplements (e.g., calcium carbonate or citrate)
- Vitamin D supplementation (cholecalciferol or calcitriol in renal insufficiency)
- Correct associated abnormalities:
- Hypomagnesemia (impairs PTH secretion and action)
- Vitamin D deficiency
- Underlying cause:
- Treat primary disorder (hypoparathyroidism, renal failure, malabsorption, etc.)
Hypercalcemia (PCa > 2.6 mmol/L)
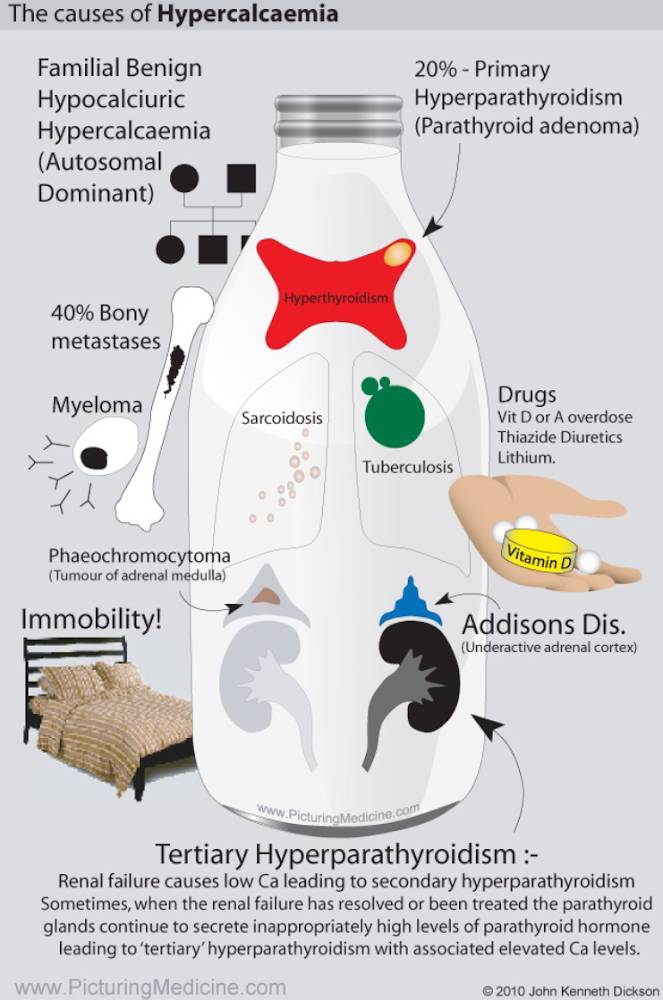
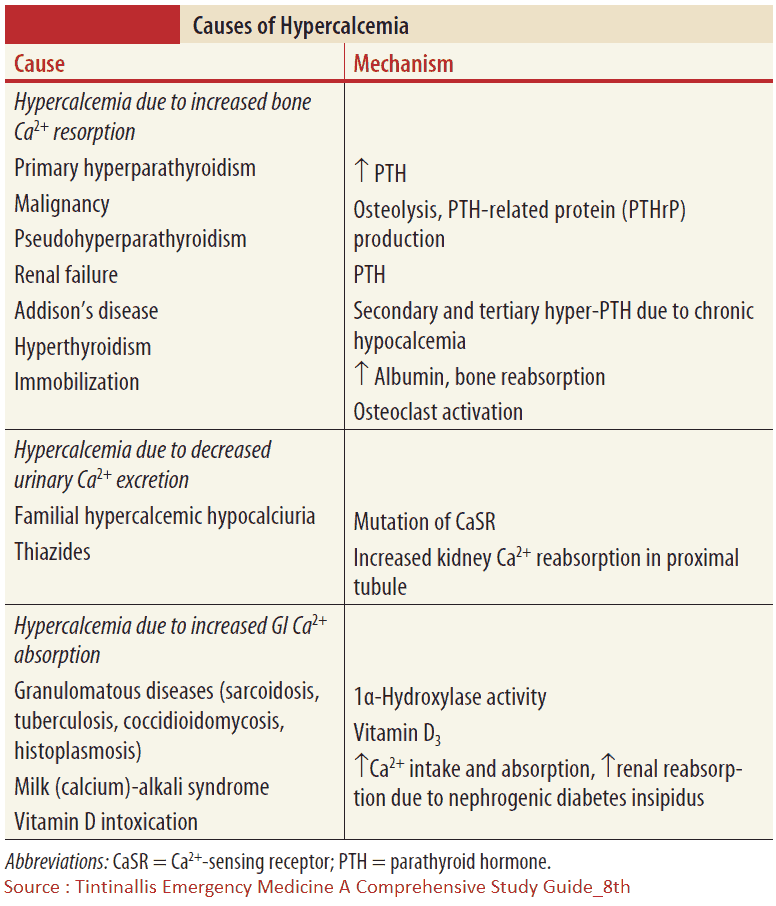
Causes of Hypercalcemia
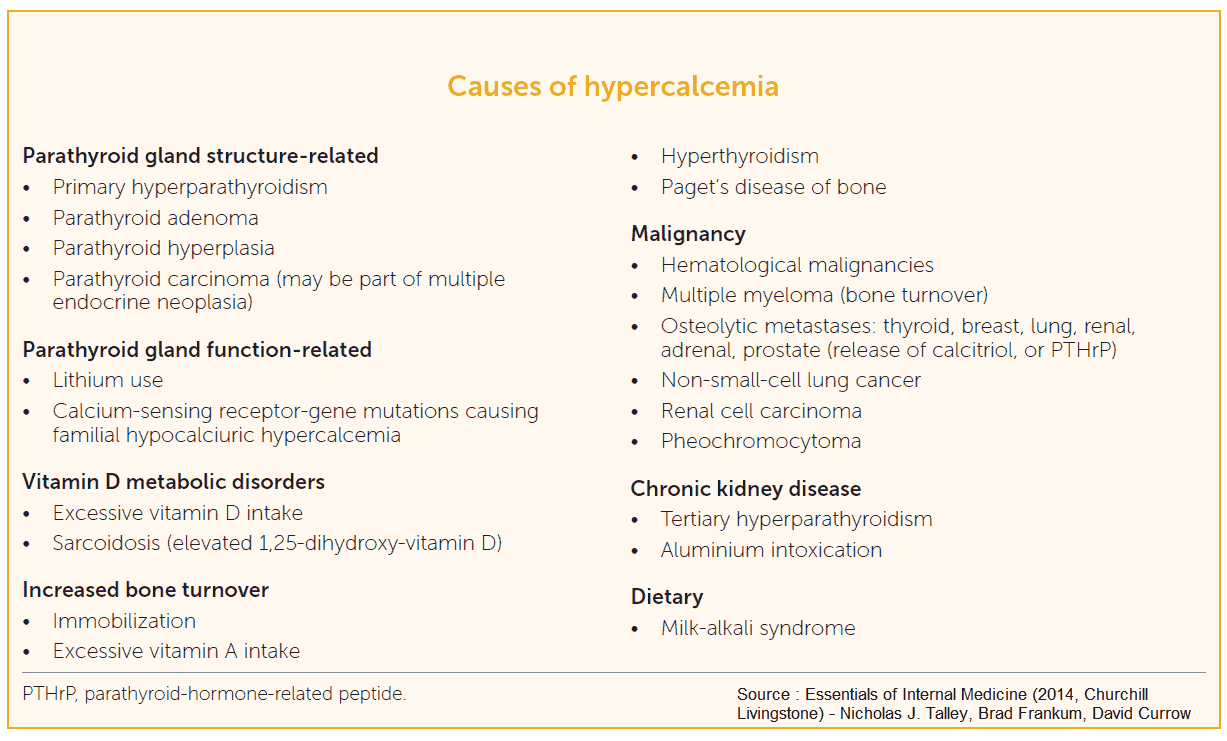
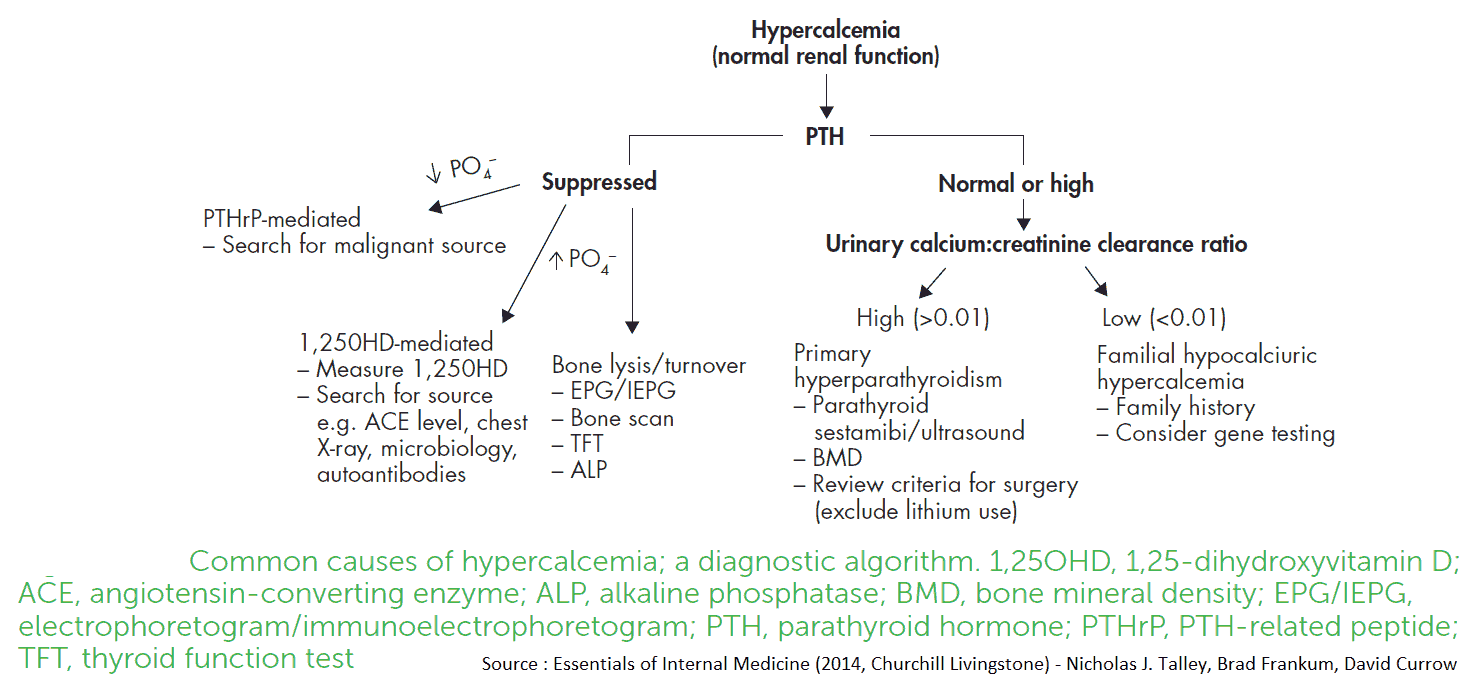
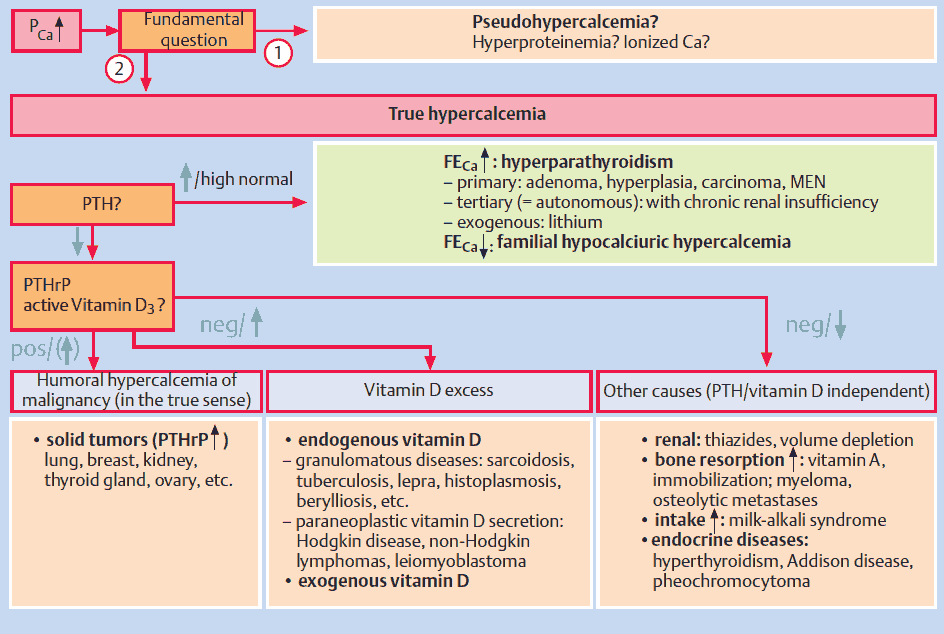
Differential Diagnosis of Hypercalcemia
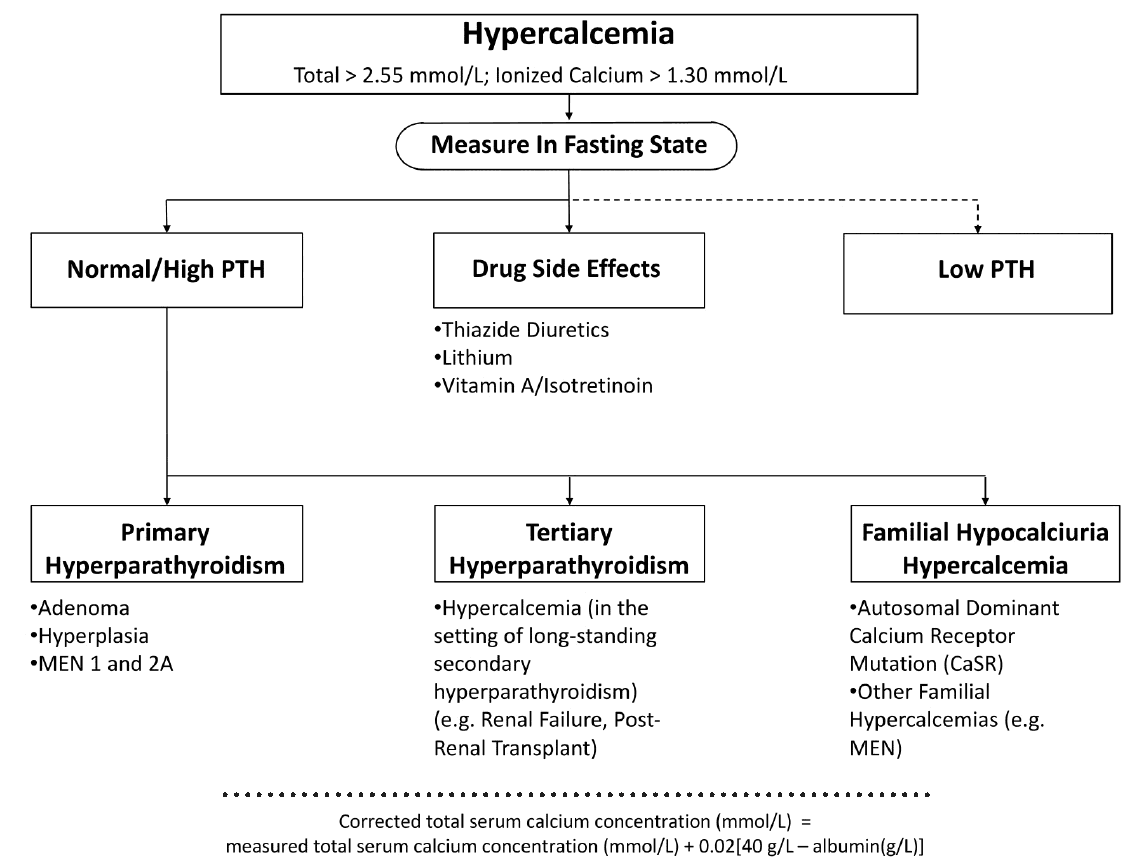
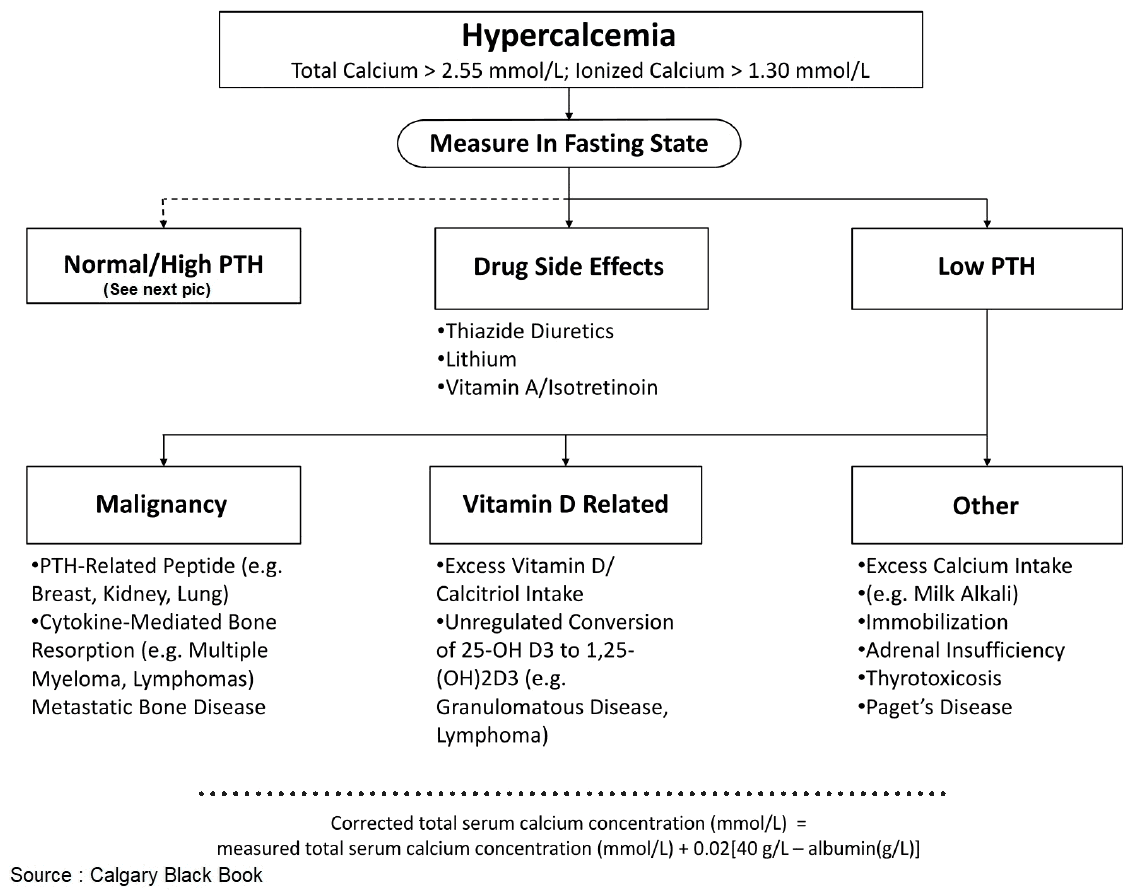
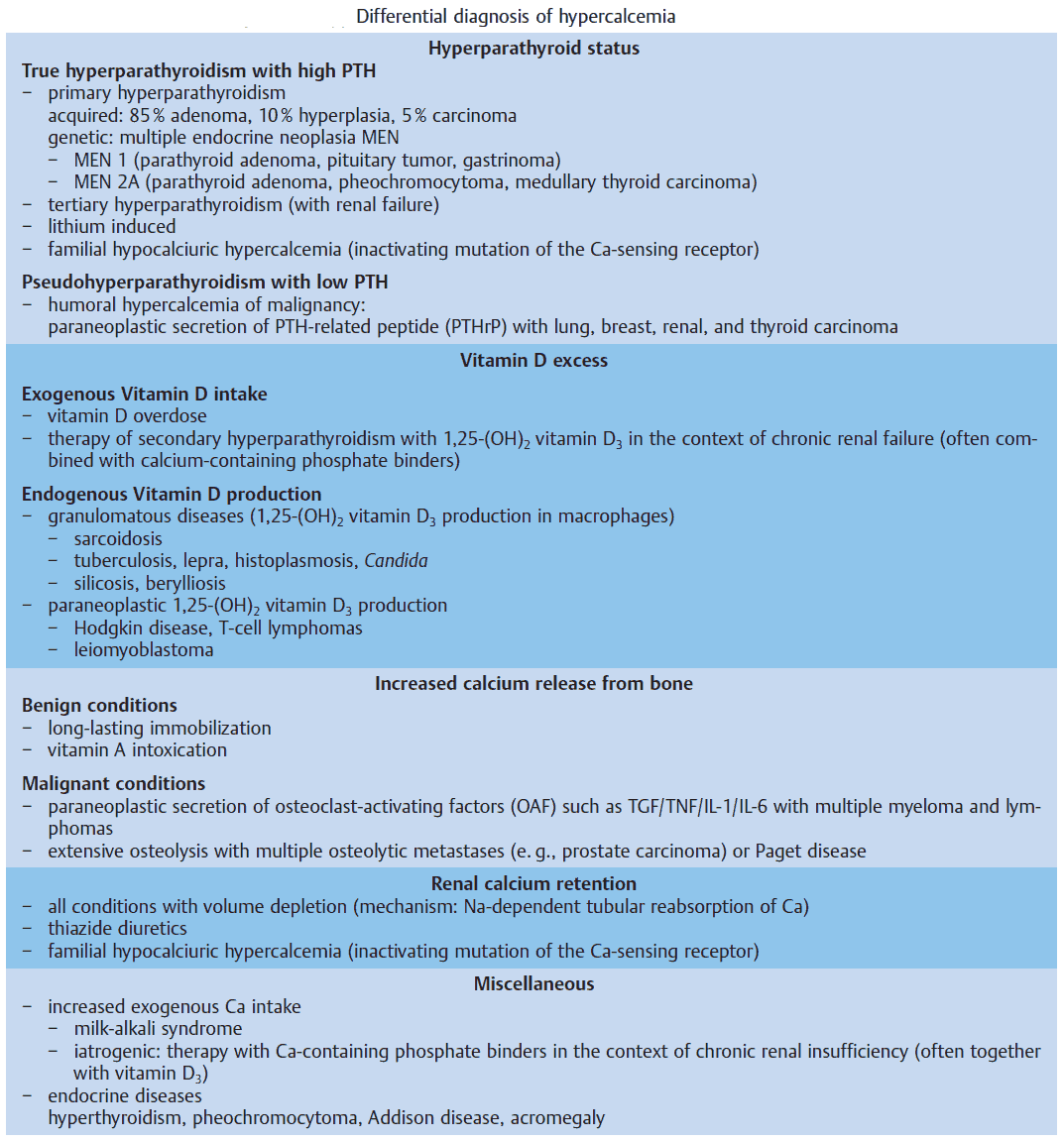
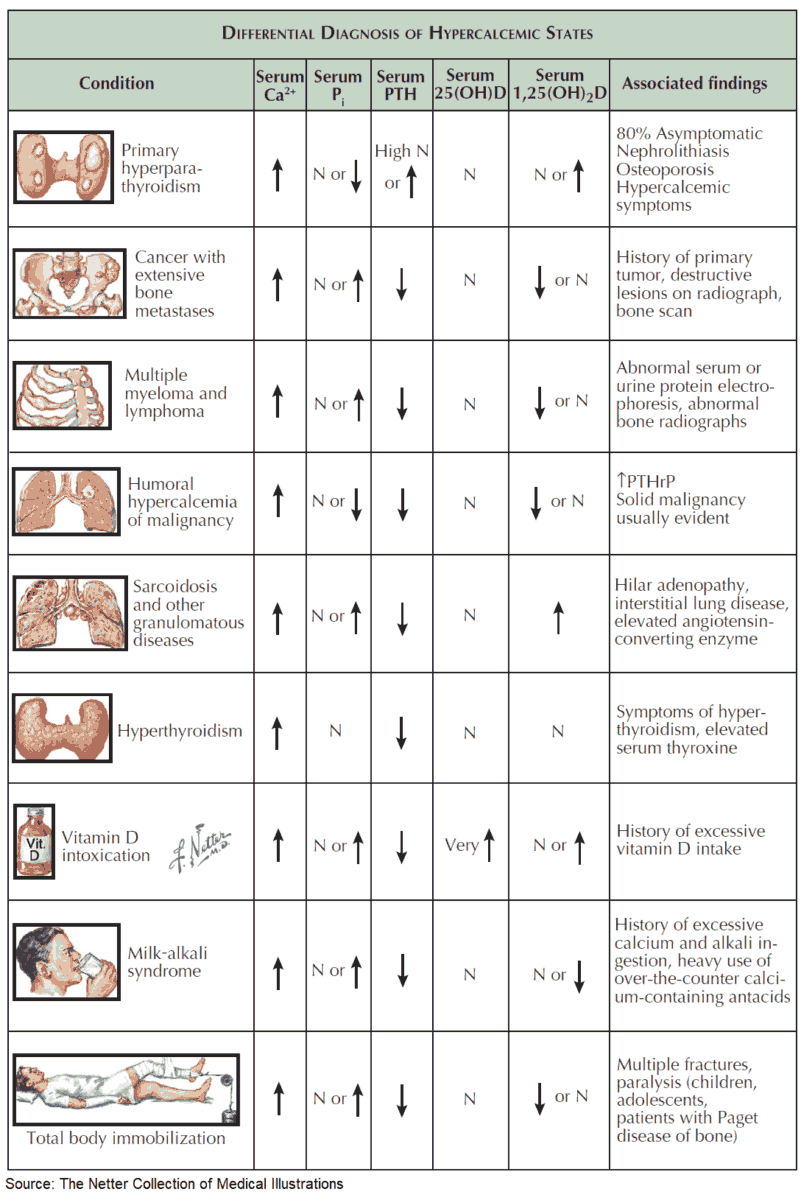
Hyperparathyroid Status
Primary hyperparathyroidism due to a benign adenoma or to diffuse hyperplasia of all four glands, and autonomous (tertiary) hyperparathyroidism in the context of long-lasting chronic renal insufficiency are frequent causes of hypercalcemia.
In contrast, genetic disorders, e. g., multiple endocrine neoplasia (MEN) or the inactivating mutation of the “calcium-sensing receptor,” which interrupts the negative feedback of serum calcium levels on PTH secretion (so-called familial hypocalciuric hypercalcemia), are rare.
The hallmarks of hyperparathyroidism are hypercalcemia, hypophosphatemia, and nephrolithiasis. In rare cases it can cause pancreatitis, ulcus duodeni, and hypertension. Bone histology shows the typical picture of fibro-osteoclasia with subperiostal resorption zones. The diagnosis is made by the measurement of serum PTH.
If hypercalcemia is observed in a patient with malignancy, but without obvious bone metastases, it is called humoral hypercalcemia of malignancy, which is usually caused by a paraneoplastic secretion of a PTH-related peptide (PTHrP). This peptide binds to the PTH receptor in the kidney and also leads to calcium mobilization from the skeleton. The secretion of PTH is suppressed by hypercalemia. It is important to distinguish this situation from patients with diffuse bone metastases, since humoral hypercalcemia is fully reversible with successful treatment of the primary tumor.
Vitamin D Excess
The most frequent cause of hypercalcemia is excessive vitamin D3 intake. It can occur, when active 1,25-(OH)2 vitamin D3 is used for treatment of secondary hyperparathyroidism in patients with renal insufficiency. Since these patients usually suffer from concomitant hyperphosphatemia, the risk of metastatic calcifications is considerable.
Other causes of vitamin D excess are the endogenous production of 1,25-(OH)2 vitamin D3 by macrophages in the context of granulomatous diseases (especially with sarcoidosis) and by tumor cells in the context of malignant lymphomas.
Increased Bone Resorption
Long-term immobilization leads to rapid calcium release from the skeleton and leads to nephrolithiasis due to hypercalciuria if hydration is insufficient (e. g., in the context of postpoliomyelitis syndrome). However, the most frequent cause of increased bone resorption are malignant diseases, either by massive and diffuse osteolytic metastases or by paraneoplastic secretion of osteoclast-activating factors, e. g., tumor necrosis factor (TNF), IL-1, IL-6, transforming growth factor (TGF). The latter can be inhibited by corticosteroid therapy.
Renal Calcium Retention
Renal tubular calcium excretion is reduced in all situations with volume depletion, since tubular reabsorption of calcium is linked to sodium reabsorption. Hypercalcemia leads to polyuria thereby worsening the volume deficit (“circulus vitiosus”).
Clinically important is the inhibition of renal calcium excretion by thiazide diuretics. It may lead to hypercalcemia, especially in the context of diuretic-induced volume depletion. On the other hand, it is used therapeutically for the treatment of nephrolithiasis with calcium-containing stones.
Other Causes
Various endocrine disorders can lead to hypercalcemia. In most cases, the precise pathogenesis is unknown. These diseases are mentioned in the image above. A particular clinical manifestation of hypercalcemiais the so-called milk-alkali syndrome. It is caused by excessive intake of milk and calcium carbonate-containingantacids, e. g., in patients with gastroduodenal ulcer disease. The hallmarks are hypercalcemia, slight hyperphosphatemia, low PTH, and metabolic alkalosis, which lead to bicarbonaturia and consecutive volume depletion and hypokalemia due to increased distal sodium delivery. The therapy consists of volume expansion and the elimination of all calcium and alkali intake.
Treatment of Hypercalcemia
- Mild / asymptomatic (<12 mg/dL or <3 mmol/L):
- Hydration and observation
- Treat underlying cause (e.g., malignancy, hyperparathyroidism, medications)
- Moderate to severe (>14 mg/dL or >3.5 mmol/L, or symptomatic):
- Volume expansion: IV isotonic saline to correct dehydration and enhance renal calcium excretion
- Promote calcium excretion: Loop diuretics (e.g., furosemide) after rehydration
- Inhibit bone resorption: IV bisphosphonates (zoledronic acid, pamidronate), denosumab in resistant cases
- Rapid reduction (temporizing): Calcitonin (short-term effect)
- Additional options: Glucocorticoids (vitamin D–mediated hypercalcemia, e.g., sarcoidosis, lymphoma)
- Refractory / life-threatening: Dialysis (especially in renal failure)
References
- Bliezikian JP, Silverberg SJ. Asymptomatic primary hyperparathyroidism. N Eng J Med 2004; 350:1746–51.
- Brown EM. Familial hypocalciuric hypercalcemia. Endocrin Metab Clin North Am 2000; 29:503–22.
- Bushinsky DA, Monk RD. Electrolyte quintet: calcium. Lancet 1998; 352:306–11.
- Carlstedt F, Lind L. Hypocalcemic syndromes. Crit Care Clin 2001; 17: 139–53.