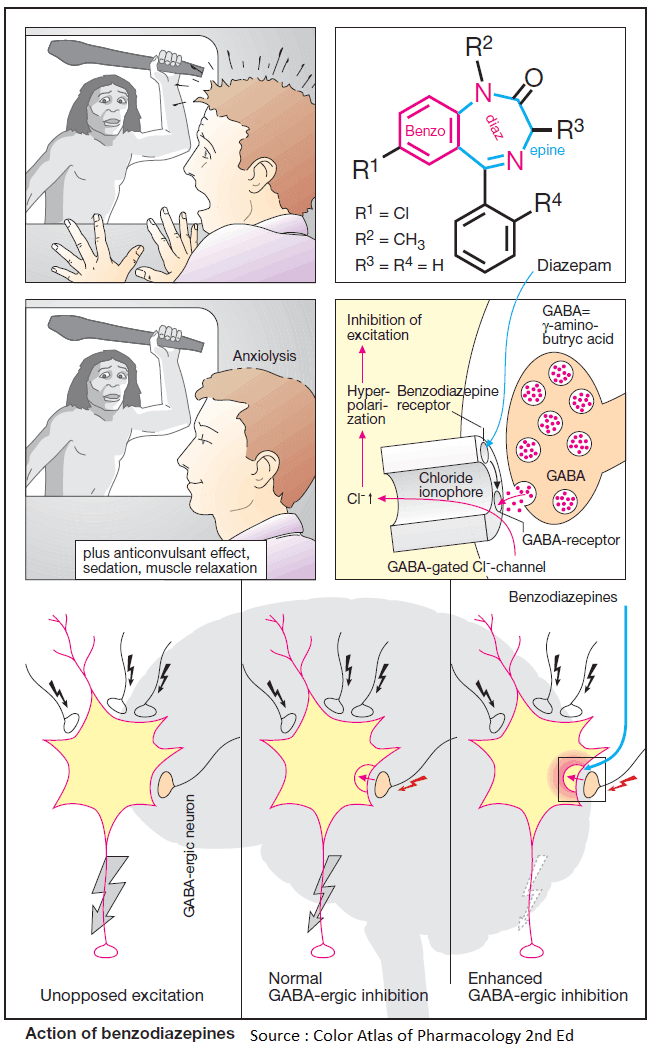
Benzodiazepines modify affective responses to sensory perceptions; specifically, they render a subject indifferent towards anxiogenic stimuli, i.e., anxiolytic action. Furthermore, benzodiazepines exert sedating, anticonvulsant, and muscle-relaxant (myotonolytic) effects.
All these actions result from augmenting the activity of inhibitory neurons and are mediated by specific benzodiazepine receptors that form an integral part of the GABAA receptor- chloride channel complex.
The inhibitory transmitter GABA acts to open the membrane chloride channels. Increased chloride conductance of the neuronal membrane effectively shortcircuits responses to depolarizing inputs.
Benzodiazepine receptor agonists increase the affinity of GABA to its receptor. At a given concentration of GABA, binding to the receptors will, therefore, be increased, resulting in an augmented response. Excitability of the neurons is diminished.
Since GABA-ergic synapses are confined to neural tissues, specific inhibition of central nervous functions can be achieved; for instance, there is little change in blood pressure, heart rate, and body temperature.
Therapeutic Indications for Benzodiazepines
- Anxiety states associated with neurotic, phobic, and depressive disorders, or myocardial infarction (decrease in cardiac stimulation due to anxiety);
- Insomnia
- Preanesthetic (preoperative) medication
- Epileptic seizures
- Hypertonia of skeletal musculature (spasticity, rigidity)
Therapeutic Index and Side Effects of Benzodiazepines
The therapeutic index of benzodiazepines, calculated with reference to the toxic dose producing respiratory depression, is greater than 100 and thus exceeds that of barbiturates and other sedative-hypnotics by more than tenfold. Benzodiazepine intoxication can be treated with a specific antidote (see below).
Since benzodiazepines depress responsivity to external stimuli, automotive driving skills and other tasks requiring precise sensorimotor coordination will be impaired.
Triazolam (t1/2 of elimination ~1.5–5.5 h) is especially likely to impair memory (anterograde amnesia) and to cause rebound anxiety or insomnia and daytime confusion. The severity of these and other adverse reactions (e.g., rage, violent hostility, hallucinations), and their increased frequency in the elderly, has led to curtailed or suspended use of triazolam in some countries (UK).
Although benzodiazepines are well tolerated, the possibility of personality changes (nonchalance, paradoxical excitement) and the risk of physical dependence with chronic use must not be overlooked.
Conceivably, benzodiazepine dependence results from a kind of habituation, the functional counterparts of which become manifest during abstinence as restlessness and anxiety; even seizures may occur. These symptoms reinforce chronic ingestion of benzodiazepines.
Benzodiazepine Antagonists
Benzodiazepine antagonists, such as flumazenil, possess affinity for benzodiazepine receptors, but they lack intrinsic activity. Flumazenil is an effective antidote in the treatment of benzodiazepine overdosage or can be used postoperatively to arouse patients sedated with a benzodiazepine.
Whereas benzodiazepines possessing agonist activity indirectly augment chloride permeability, inverse agonists exert an opposite action. These substances give rise to pronounced restlessness, excitement, anxiety, and convulsive seizures. There is, as yet, no therapeutic indication for their use.
Pharmacokinetics of Benzodiazepines
All benzodiazepines exert their actions at specific receptors. The choice between different agents is dictated by their speed, intensity, and duration of action. These, in turn, reflect physicochemical and pharmacokinetic properties.
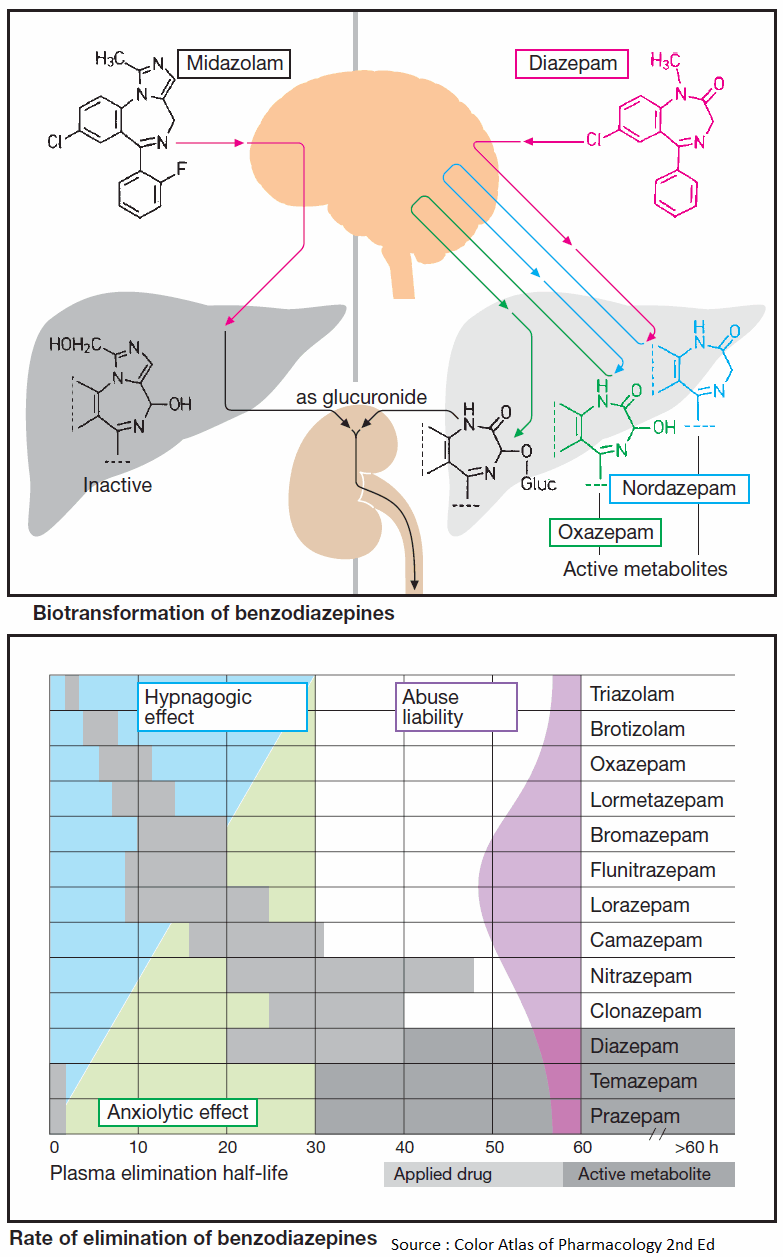
Individual benzodiazepines remain in the body for very different lengths of time and are chiefly eliminated through biotransformation. Inactivation may entail a single chemical reaction or several steps (e.g., diazepam) before an inactive metabolite suitable for renal elimination is formed.
Since the intermediary products may, in part, be pharmacologically active and, in part, be excreted more slowly than the parent substance, metabolites will accumulate with continued regular dosing and contribute significantly to the final effect.
Biotransformation begins either at substituents on the diazepine ring (diazepam: N-dealkylation at position 1; midazolam: hydroxylation of the methyl group on the imidazole ring) or at the diazepine ring itself.
Hydroxylated midazolam is quickly eliminated following glucuronidation (t1/2 ~ 2 h). N-demethyldiazepam (nordazepam) is biologically active and undergoes hydroxylation at position 3 on the diazepine ring. The hydroxylated product (oxazepam) again is pharmacologically active.
By virtue of their long half-lives, diazepam (t1/2 ~ 32 h) and, still more so, its metabolite, nordazepam (t1/2 50–90 h), are eliminated slowly and accumulate during repeated intake.
Oxazepam undergoes conjugation to glucuronic acid via its hydroxyl group (t1/2 = 8 h) and renal excretion.
The range of elimination half-lives for different benzodiazepines or their active metabolites is represented by the shaded areas in this Image. Substances with a short half-life that are not converted to active metabolites can be used for induction or maintenance of sleep (light blue area in B in image).
Substances with a long half-life are preferable for long-term anxiolytic treatment (light green area) because they permit maintenance of steady plasma levels with single daily dosing.
Midazolam enjoys use by the i.v. route in preanesthetic medication and anesthetic combination regimens.
Benzodiazepine Dependence and Abstinence Syndrome
Prolonged regular use of benzodiazepines can lead to physical dependence. With the long-acting substances marketed initially, this problem was less obvious in comparison with other dependence- producing drugs because of the delayed appearance of withdrawal symptoms.
The severity of the abstinence syndrome is inversely related to the elimination t1/2, ranging from mild to moderate (restlessness, irritability, sensitivity to sound and light, insomnia, and tremulousness) to dramatic (depression, panic, delirium, grand mal seizures).
Some of these symptoms pose diagnostic difficulties, being indistinguishable from the ones originally treated. Administration of a benzodiazepine antagonist would abruptly provoke abstinence signs. There are indications that substances with intermediate elimination half-lives are most frequently abused (violet area in B in Image).