Table of Contents
In the area of a gastric or duodenal peptic ulcer, the mucosa has been attacked by digestive juices to such an extent as to expose the subjacent connective tissue layer (submucosa).
This self-digestion occurs when the equilibrium between the corrosive hydrochloric acid and acid-neutralizing mucus, which forms a protective cover on the mucosal surface, is shifted in favor of hydrochloric acid. Mucosal damage can be promoted by Helicobacter pylori bacteria that colonize the gastric mucus.
READ MORE: From Heartburn to Ulcers: Understanding Peptic Ulcer Disease
Drugs are employed with the following therapeutic aims:
- to relieve pain
- to accelerate healing
- to prevent ulcer recurrence
Therapeutic approaches are threefold:
- to reduce aggressive forces by lowering H+ output
- to increase protective forces by means of mucoprotectants
- to eradicate Helicobacter pylori
Drugs for Lowering Acid Concentration
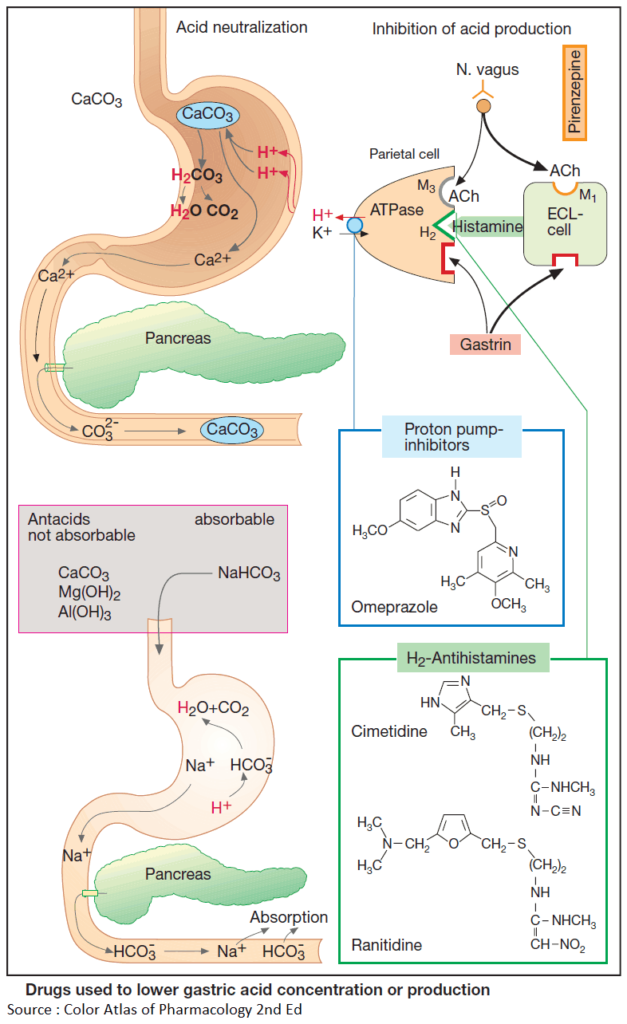
Acid neutralization (Antacids)
H+-binding groups such as CO3 2–, HCO3 – or OH–, together with their counter ions, are contained in antacid drugs. Neutralization reactions occurring after intake of CaCO3 and NaHCO3, respectively, are shown in this Image.
With nonabsorbable antacids, the counter ion is dissolved in the acidic gastric juice in the process of neutralization. Upon mixture with the alkaline pancreatic secretion in the duodenum, it is largely precipitated again by basic groups, e.g., as CaCO3 or AlPO4, and excreted in feces.
Therefore, systemic absorption of counter ions or basic residues is minor. In the presence of renal insufficiency, however, absorption of even small amounts may cause an increase in plasma levels of counter ions (e.g., magnesium intoxication with paralysis and cardiac disturbances).
Side effects of Antacids
- Reduced absorption of other drugs
- Hypophosphatemia (phosphate depletion of the body with excessive intake of Al(OH)3)
- Hypermagnesemia (in renal insufficiency)
- Diarrhea (Mg(OH)2 antacids)
- Constipation (Al(OH)3 antacids)
Precipitation in the gut lumen is responsible for other side effects, such as reduced absorption of other drugs due to their adsorption to the surface of precipitated antacid or, phosphate depletion of the body with excessive intake of Al(OH)3.
Na+ ions remain in solution even in the presence of HCO3 –-rich pancreatic secretions and are subject to absorption, like HCO3 –. Because of the uptake of Na+, use of NaHCO3 must be avoided in conditions requiring restriction of NaCl intake, such as hypertension, cardiac failure, and edema.
Since food has a buffering effect, antacids are taken between meals (e.g., 1 and 3 h after meals and at bedtime). Nonabsorbable antacids are preferred.
Because Mg(OH)2 produces a laxative effect (cause: osmotic action, release of cholecystokinin by Mg2+, or both) and Al(OH)3 produces constipation (cause: astringent action of Al3+), these two antacids are frequently used in combination.
Inhibitors of Acid Production
Acting on their respective receptors, the transmitter acetylcholine, the hormone gastrin, and histamine released intramucosally stimulate the parietal cells of the gastric mucosa to increase output of HCl.
Histamine comes from enterochromaffin- like (ECL) cells; its release is stimulated by the vagus nerve (via M1 receptors) and hormonally by gastrin. The effects of acetylcholine and histamine can be abolished by orally applied antagonists that reach parietal cells via the blood.
Cholinoceptor Antagonists (Anticholinergics)
The cholinoceptor antagonist pirenzepine, unlike atropine, prefers cholinoceptors of the M1 type, does not penetrate into the CNS, and thus produces fewer atropine-like side effects. The cholinoceptors on parietal cells probably belong to the M3 subtype. Hence, pirenzepine may act by blocking M1 receptors on ECL cells or submucosal neurons.
H2-antihistamines
Histamine receptors on parietal cells belong to the H2 type and are blocked by H2-antihistamines. Because histamine plays a pivotal role in the activation of parietal cells, H2-antihistamines also diminish responsivity to other stimulants, e.g., gastrin (in gas- trin-producing pancreatic tumors, Zollinger- Ellison syndrome).
Cimetidine, the first H2-antihistamine used therapeutically, only rarely produces side effects (CNS disturbances such as confusion; endocrine effects in the male, such as gynecomastia, decreased libido, impotence).
Unlike cimetidine, its newer and more potent congeners, ranitidine, nizatidine, and famotidine, do not interfere with the hepatic biotransformation of other drugs.
Proton Pump Inhibitors
Omeprazole can cause maximal inhibition of HCl secretion. Given orally in gastric juice-resistant capsules, it reaches parietal cells via the blood. In the acidic milieu of the mucosa, an active metabolite is formed and binds covalently to the ATP-driven proton pump (H+/K+ ATPase) that transports H+ in exchange for K+ into the gastric juice.
Lansoprazole and pantoprazole produce analogous effects. The proton pump inhibitors are first-line drugs for the treatment of gastroesophageal reflux disease.
Mucosal Protective Drugs
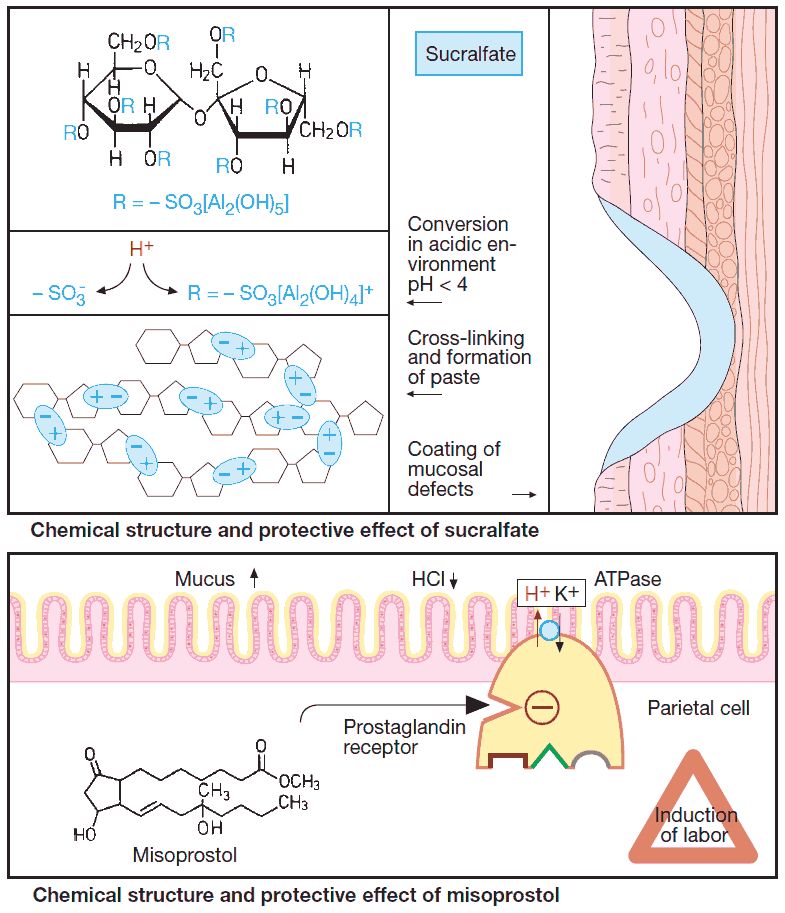
Sucralfate
Sucralfate contains numerous aluminum hydroxide residues. However, it is not an antacid because it fails to lower the overall acidity of gastric juice.
After oral intake, sucralfate molecules undergo cross-linking in gastric juice, forming a paste that adheres to mucosal defects and exposed deeper layers. Here sucralfate intercepts H+. Protected from acid, and also from pepsin, trypsin, and bile acids, the mucosal defect can heal more rapidly.
Sucralfate is taken on an empty stomach (1 h before meals and at bedtime). It is well tolerated; however, released Al3+ ions can cause constipation.
Misoprostol
Misoprostol is a semisynthetic prostaglandin derivative with greater stability than natural prostaglandin, permitting absorption after oral administration. Like locally released prostaglandins, it promotes mucus production and inhibits acid secretion.
Additional systemic effects (frequent diarrhea; risk of precipitating contractions of the gravid uterus) significantly restrict its therapeutic utility.
Carbenoxolone
Carbenoxolone is a derivative of glycyrrhetinic acid, which occurs in the sap of licorice root (succus liquiritiae). Carbenoxolone stimulates mucus production. At the same time, it has a mineralocorticoid-like action (due to inhibition of 11-β-hydroxysteroid dehydrogenase) that promotes renal reabsorption of NaCl and water. It may, therefore, exacerbate hypertension, congestive heart failure, or edemas. It is obsolete.
Eradication of Helicobacter pylori C
This microorganism plays an important role in the pathogenesis of chronic gastritis and peptic ulcer disease. The combination of antibacterial drugs and omeprazole has proven effective.
In case of intolerance to amoxicillin or clarithromycin, metronidazole can be used as a substitute. Colloidal bismuth compounds are also effective; however, the problem of heavy-metal exposure compromises their long-term use.