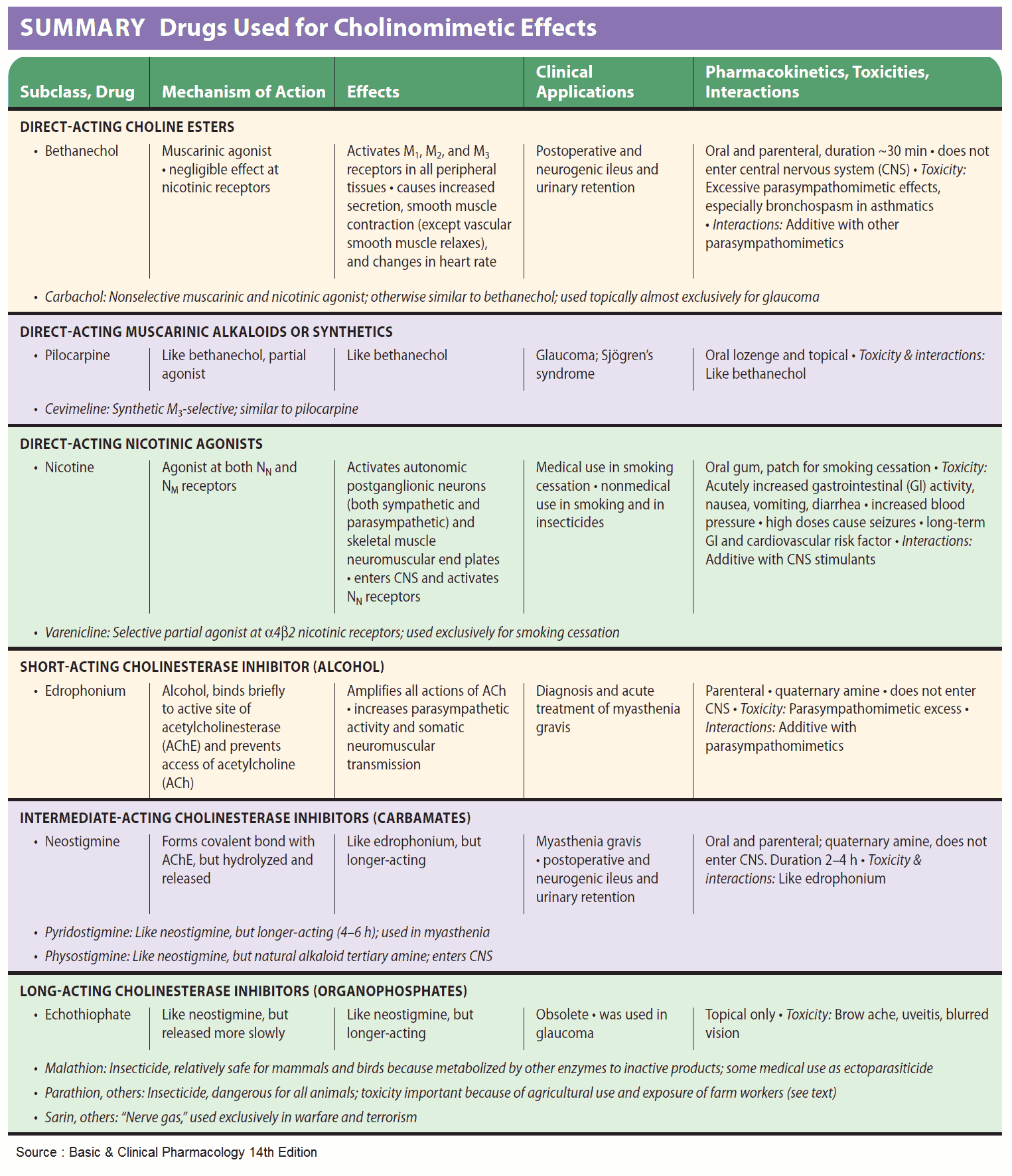
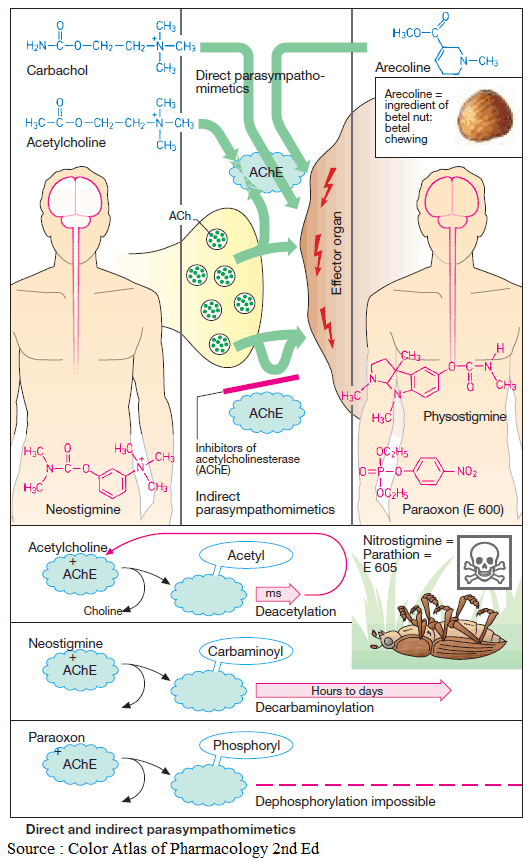
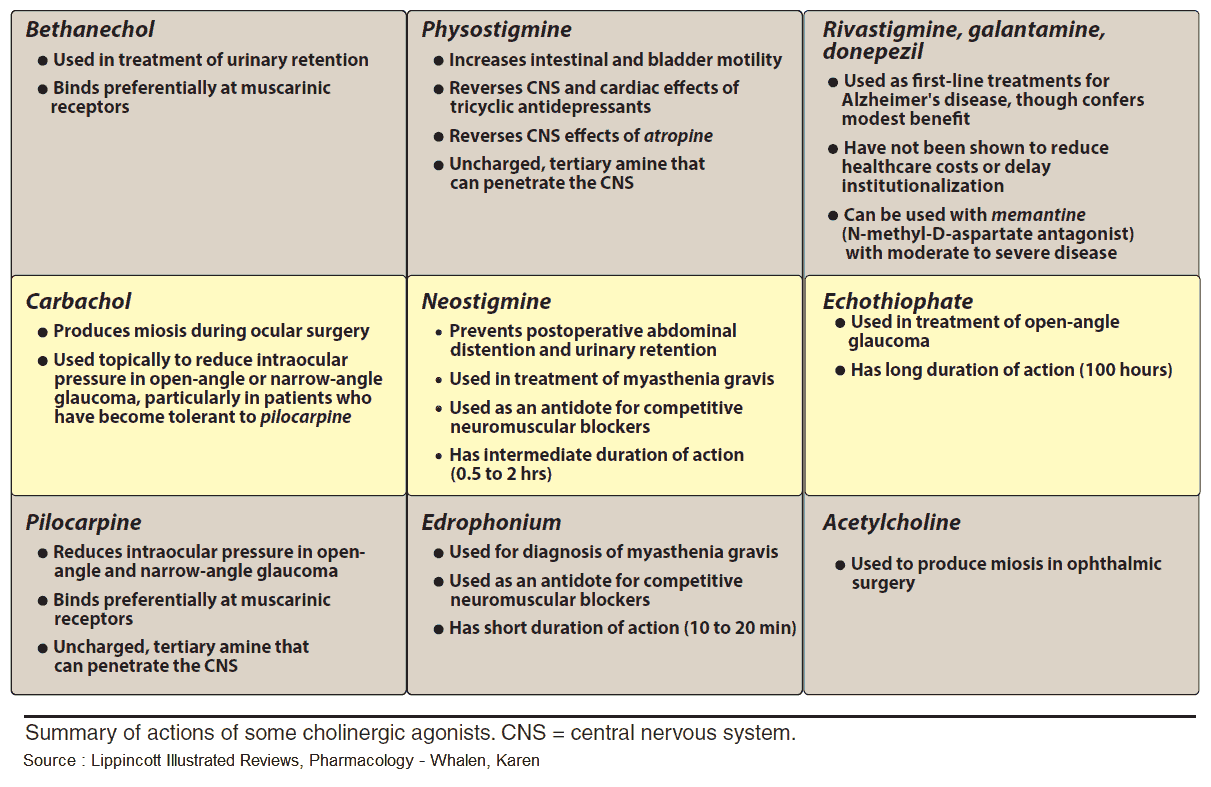
Acetylcholine (ACh) is too rapidly hydrolyzed and inactivated by acetylcholinesterase (AChE) to be of any therapeutic use; however, its action can be mimicked by other substances, namely direct or indirect parasympathomimetics (cholinomimetics).
Direct Parasympathomimetics
The choline ester, carbachol, activates M-cholinoceptors, but is not hydrolyzed by acetylcholinesterase (AChE). Carbachol can thus be effectively employed for local application to the eye (glaucoma) and systemic administration (bowel atonia, bladder atonia).
The alkaloids, pilocarpine (from Pilocarpus jaborandi) and arecoline (from Areca catechu; betel nut) also act as direct parasympathomimetics. As tertiary amines, they moreover exert central effects. The central effect of muscarinelike substances consists of an enlivening, mild stimulation that is probably the effect desired in betel chewing, a widespread habit in South Asia. Of this group, only pilocarpine enjoys therapeutic use, which is limited to local application to the eye in glaucoma.
Indirect Parasympathomimetics
Acetylcholinesterase (AChE) can be inhibited selectively, with the result that ACh released by nerve impulses will accumulate at cholinergic synapses and cause prolonged stimulation of cholinoceptors. Inhibitors of acetylcholinesterase (AChE) are, therefore, indirect parasympathomimetics.
Their action is evident at all cholinergic synapses. Chemically, these agents include esters of carbamic acid (carbamates such as physostigmine, neostigmine) and of phosphoric acid (organophosphates such as paraoxon = E600 and nitrostigmine = parathion = E605, its prodrug).
Members of both groups react like ACh with AChE and can be considered false substrates. The esters are hydrolyzed upon formation of a complex with the enzyme. The rate-limiting step in ACh hydrolysis is deacetylation of the enzyme, which takes only milliseconds, thus permitting a high turnover rate and activity of AChE.
Decarbaminoylation following hydrolysis of a carbamate takes hours to days, the enzyme remaining inhibited as long as it is carbaminoylated. Cleavage of the phosphate residue, i.e. dephosphorylation, is practically impossible; enzyme inhibition is irreversible.
Uses of Parasympathomimetics (Cholinomimetics)
The quaternary carbamate neostigmine is employed as an indirect parasympathomimetic in postoperative atonia of the bowel or bladder. Furthermore, it is needed to overcome the relative ACh-deficiency at the motor endplate in myasthenia gravis or to reverse the neuromuscular blockade caused by nondepolarizing muscle relaxants (decurarization before discontinuation of anesthesia).
The tertiary carbamate physostigmine can be used as an antidote in poisoning with parasympatholytic drugs, because it has access to AChE in the brain.
Carbamates (neostigmine, pyridostigmine, physostigmine) and organophosphates (paraoxon, ecothiopate) can also be applied locally to the eye in the treatment of glaucoma; however, their long-term use leads to cataract formation.
Agents from both classes also serve as insecticides. Although they possess high acute toxicity in humans, they are more rapidly degraded than is DDT following their emission into the environment. Tacrine is not an ester and interferes only with the choline-binding site of AChE. It is effective in alleviating symptoms of dementia in some subtypes of Alzheimer’s disease.