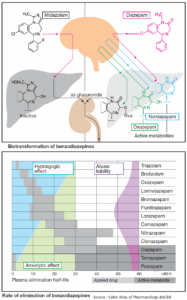
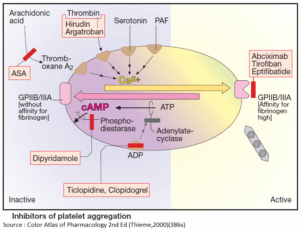
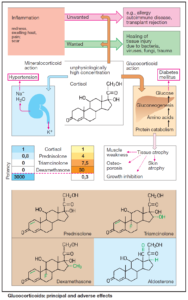
Table of Contents
Cell Wall of Bacteria
In most bacteria, a cell wall surrounds the cell like a rigid shell that protects against noxious outside influences and prevents rupture of the plasma membrane from a high internal osmotic pressure.
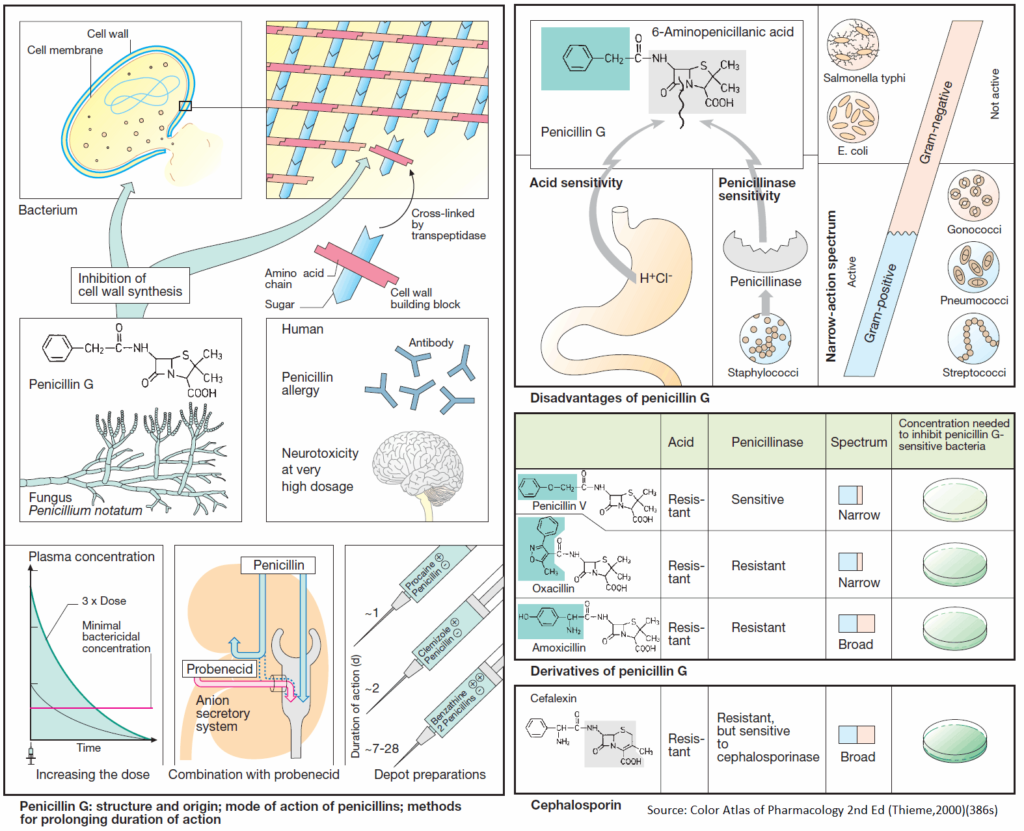
The structural stability of the cell wall is due mainly to the murein (peptidoglycan) lattice. This consists of basic building blocks linked together to form a large macromolecule. Each basic unit contains the two linked aminosugars N-acetylglucosamine and N-acetylmuramyl acid; the latter bears a peptide chain.
The building blocks are synthesized in the bacterium, transported outward through the cell membrane, and assembled as illustrated schematically. The enzyme transpeptidase cross-links the peptide chains of adjacent aminosugar chains.
Inhibitors of cell wall synthesis
Inhibitors of cell wall synthesis are suitable antibacterial agents, because animal and human cells lack a cell wall. They exert a bactericidal action on growing or multiplying germs. Members of this class include:
- β-lactam antibiotics
- penicillins
- cephalosporinsin
- bacitracin
- vancomycin
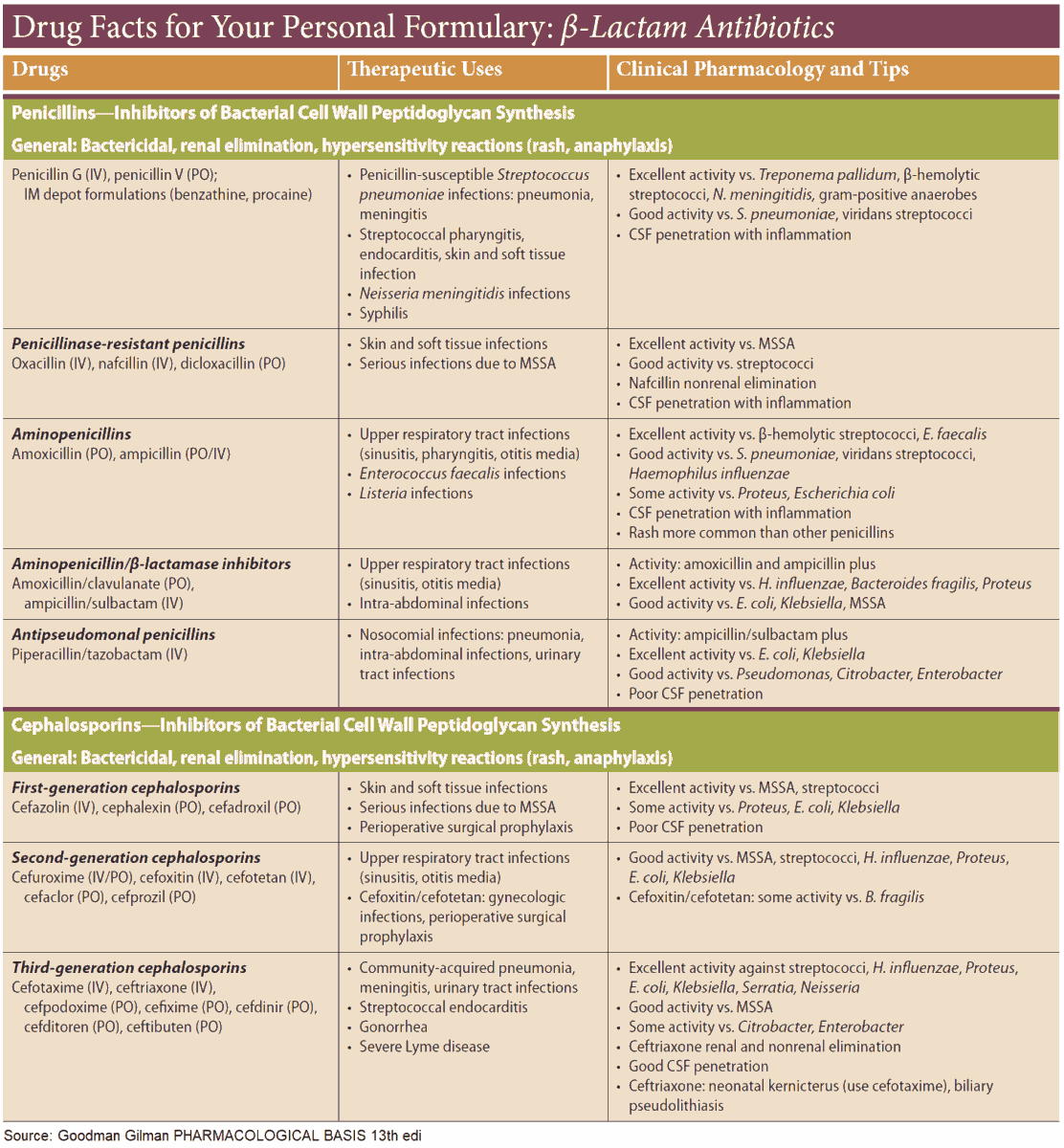
Penicillins
The parent substance of this group is penicillin G (benzylpenicillin). It is obtained from cultures of mold fungi, originally from Penicillium notatum. Penicillin G contains the basic structure common to all penicillins, 6-amino-penicillanic acid (6-APA), comprised of a thiazolidine and a 4-membered β-lactam ring. 6- APA itself lacks antibacterial activity.
Penicillins disrupt cell wall synthesis by inhibiting transpeptidase. When bacteria are in their growth and replication phase, penicillins are bactericidal; due to cell wall defects, the bacteria swell and burst.
SIDE EFFECTS: Penicillins are generally well tolerated; with penicillin G, the daily dose can range from approx. 0.6 g i.m. (= 106 international units, 1 Mega I.U.) to 60 g by infusion. The most important adverse effects are due to hypersensitivity (incidence up to 5%), with manifestations ranging from skin eruptions to anaphylactic shock (in less than 0.05% of patients). Known penicillin allergy is a contraindication for these drugs. Because of an increased risk of sensitization, penicillins must not be used locally. Neurotoxic effects, mostly convulsions due to GABA antagonism, may occur if the brain is exposed to extremely high concentrations, e.g., after rapid i.v. injection of a large dose or intrathecal injection.
Penicillin G undergoes rapid renal elimination mainly in unchanged form (plasma t1/2 ~ 0.5 h). The duration of the effect can be prolonged by:
- Use of higher doses, enabling plasma levels to remain above the minimally effective antibacterial concentration
- Combination with probenecid.
- Renal elimination of penicillin occurs chiefly via the anion (acid)-secretory system of the proximal tubule (-COOH of 6-APA). The acid probenecid competes for this route and thus retards penicillin elimination
- Intramuscular administration in depot form.
- In its anionic form (-COO-) penicillin G forms poorly water-soluble salts with substances containing a positively charged amino group (procaine; clemizole, an antihistamine; benzathine, dicationic). Depending on the substance, release of penicillin from the depot occurs over a variable interval.
DISADVANTAGES: Although very well tolerated, penicillin G has disadvantages that limit its therapeutic usefulness:
- It is inactivated by gastric acid, which cleaves the β-lactam ring, necessitating parenteral administration.
- The β-lactam ring can also be opened by bacterial enzymes (β-lactamases); in particular, penicillinase, which can be produced by staphylococcal strains, renders them resistant to penicillin G.
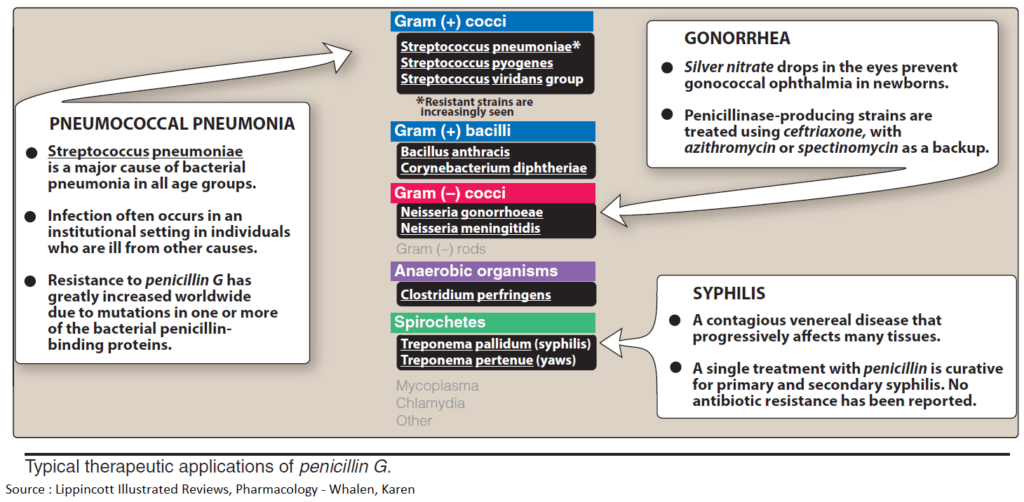
- The antibacterial spectrum is narrow; although it encompasses many gram-positive bacteria, gram-negative cocci, and spirochetes, many gram-negative pathogens are unaffected.
Derivates of Penicillin G
Derivatives with a different substituent on 6-APA possess advantages:
- Acid resistance permits oral administration, provided that enteral absorption is possible. Penicillin V (phenoxymethylpenicillin) exhibits antibacterial properties similar to those of penicillin G.
- Due to their penicillinase resistance, isoxazolylpenicillins (oxacillin dicloxacillin, flucloxacillin) are suitable for the (oral) treatment of infections caused by penicillinaseproducing staphylococci.
- Extended activity spectrum: The aminopenicillin amoxicillin is active against many gramnegative organisms, e.g., coli bacteria or Salmonella typhi. It can be protected from destruction by penicillinase by combination with inhibitors of penicillinase (clavulanic acid, sulbactam, tazobactam).
The structurally close congener ampicillin (no 4-hydroxy group) has a similar activity spectrum. However, because it is poorly absorbed (<50%) and therefore causes more extensive damage to the gut microbial flora (side effect: diarrhea), it should be given only by injection.
A still broader spectrum (including Pseudomonas bacteria) is shown by carboxypenicillins (carbenicillin, ticarcillin) and acylaminopenicillins (mezclocillin, azlocillin, piperacillin). These substances are neither acid stable nor penicillinase resistant.
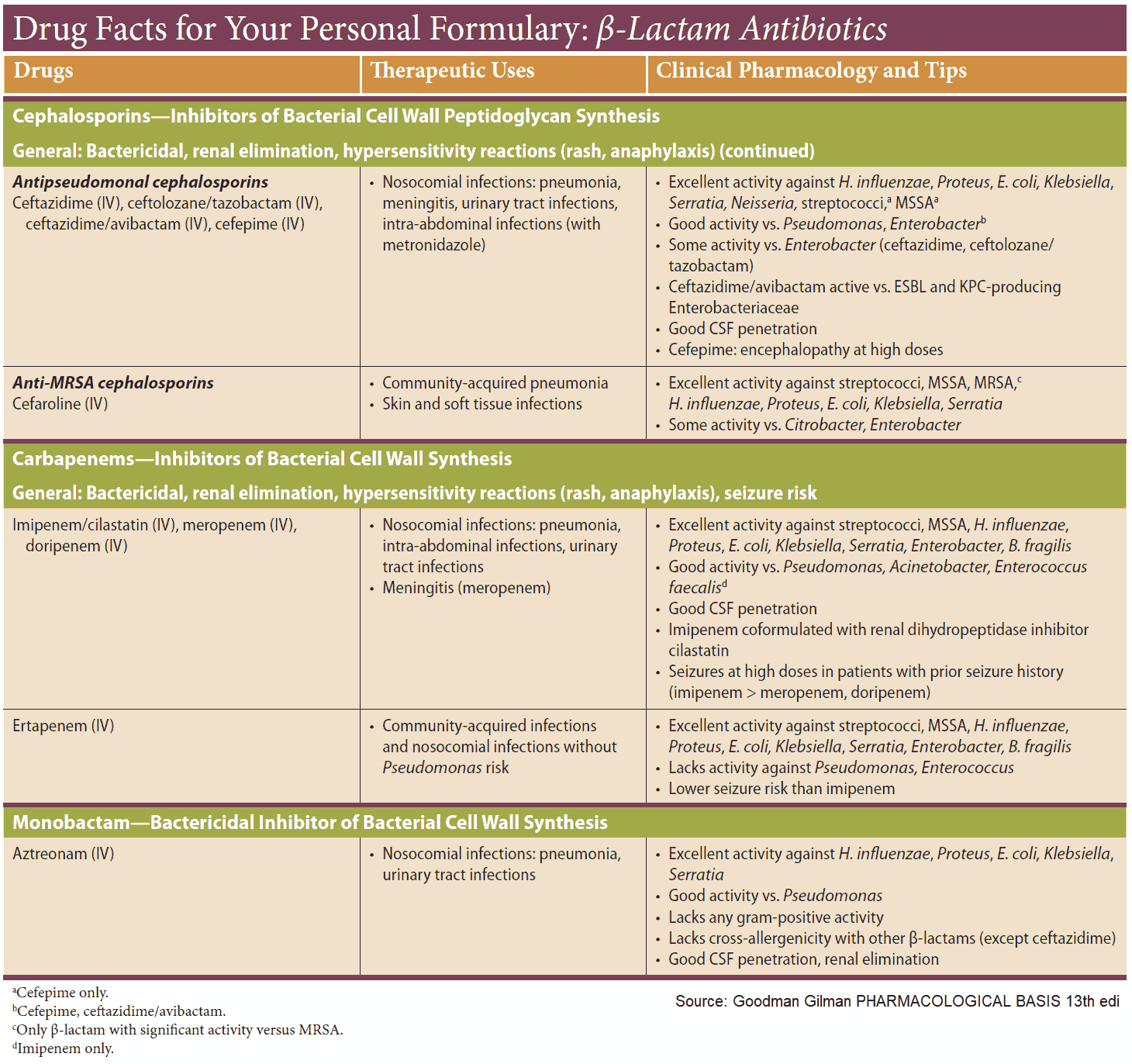
Cephalosporins
These β-lactam antibiotics are also fungal products and have bactericidal activity due to inhibition of transpeptidase. Their shared basic structure is 7-aminocephalosporanic acid, as exemplified by cephalexin.
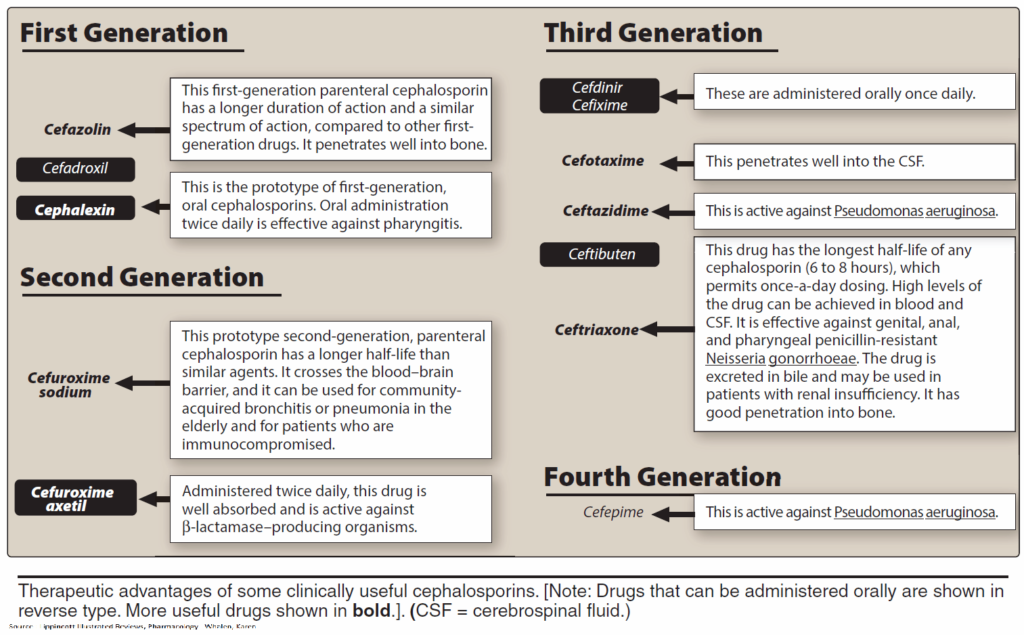
Cephalosporins are acid stable, but many are poorly absorbed. Because they must be given parenterally, most—including those with high activity—are used only in clinical settings. A few, e.g., cephalexin, are suitable for oral use.
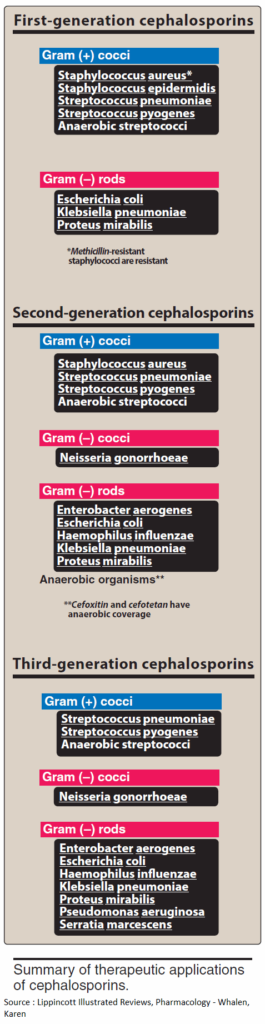
Cephalosporins are penicillinase-resistant, but cephalosporinase-forming organisms do exist. Some derivatives are, however, also resistant to this β-lactamase. Cephalosporins are broad-spectrum antibacterials.
Newer derivatives (e.g., cefotaxime, cefmenoxin, cefoperazone, ceftriaxone, ceftazidime, moxalactam) are also effective against pathogens resistant to various other antibacterials. Cephalosporins are mostly well tolerated. All can cause allergic reactions, some also renal injury, alcohol intolerance, and bleeding (vitamin K antagonism).
Other inhibitors of cell wall synthesis
Bacitracin and vancomycin interfere with the transport of peptidoglycans through the cytoplasmic membrane and are active only against gram-positive bacteria.
- Bacitracin is a polypeptide mixture, markedly nephrotoxic and used only topically.
- Vancomycin is a glycopeptide and the drug of choice for the (oral) treatment of bowel inflammations occurring as a complication of antibiotic therapy (pseudomembranous enterocolitis caused by Clostridium difficile). It is not absorbed.