Table of Contents
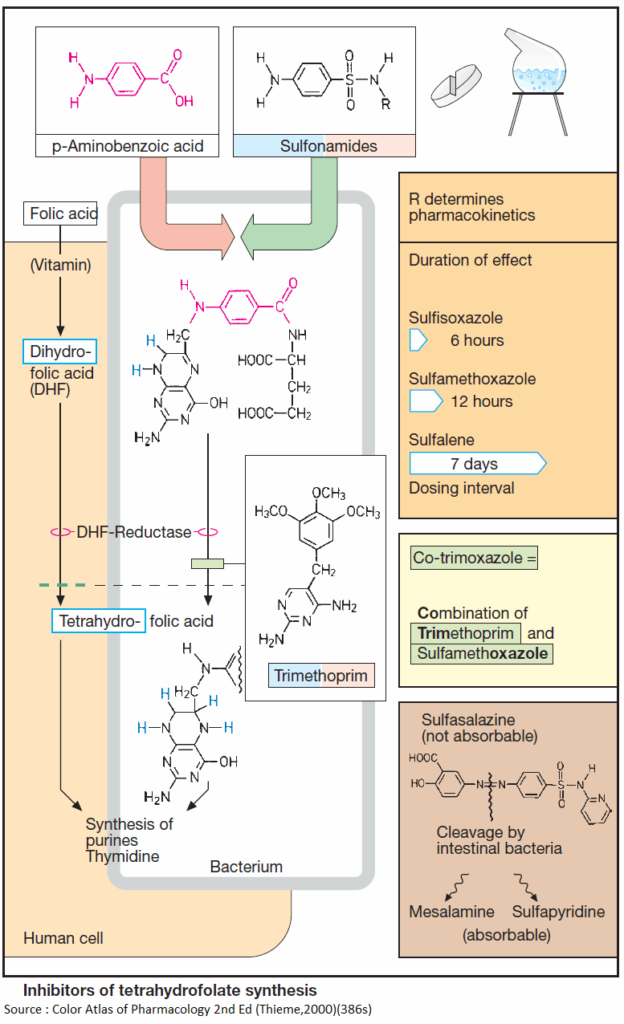
Tetrahydrofolic acid (THF)
Tetrahydrofolic acid (THF) or Tetrahydrofolate is a co-enzyme in the synthesis of purine bases and thymidine. These are constituents of DNA and RNA and required for cell growth and replication. Lack of THF leads to inhibition of cell proliferation.
Formation of THF from dihydrofolate (DHF) is catalyzed by the enzyme dihydrofolate reductase. DHF is made from folic acid, a vitamin that cannot be synthesized in the body, but must be taken up from exogenous sources.
Most bacteria do not have a requirement for folate, because they are capable of synthesizing folate, more precisely dihydrofolate DHF, from precursors. Selective interference with bacterial synthesis of Tetrahydrofolate (THF) can be achieved with sulfonamides and trimethoprim.
Sulfonamides
Sulfonamides structurally resemble p-aminobenzoic acid (PABA), a precursor in bacterial DHF synthesis. As false substrates, sulfonamides competitively inhibit utilization of PABA, hence DHF synthesis. Because most bacteria cannot take up exogenous folate, they are depleted of DHF.
Sulfonamides thus possess bacteriostatic activity against a broad spectrum of pathogens. Sulfonamides are produced by chemical synthesis. The basic structure is shown in (A). Residue R determines the pharmacokinetic properties of a given sulfonamide.
Most sulfonamides are well absorbed via the enteral route. They are metabolized to varying degrees and eliminated through the kidney. Rates of elimination, hence duration of effect, may vary widely. Some members are poorly absorbed from the gut and are thus suitable for the treatment of bacterial bowel infections.
Adverse effects may include, among others, allergic reactions, sometimes with severe skin damage, displacement of other plasma protein-bound drugs or bilirubin in neonates (danger of kernicterus, hence contraindication for the last weeks of gestation and in the neonate).
Because of the frequent emergence of resistant bacteria, sulfonamides are now rarely used. Introduced in 1935, they were the first broad-spectrum chemotherapeutics.
Trimethoprim
Trimethoprim inhibits bacterial DHF reductase, the human enzyme being significantly less sensitive than the bacterial one (rarely bone marrow depression). A 2,4-diaminopyrimidine, trimethoprim, has bacteriostatic activity against a broad spectrum of pathogens. It is used mostly as a component of cotrimoxazole.
Co-trimoxazole
Co-trimoxazole is a combination of trimethoprim and the sulfonamide sulfamethoxazole. Since THF synthesis is inhibited at two successive steps, the antibacterial effect of co-trimoxazole is better than that of the individual components. Resistant pathogens are infrequent; a bactericidal effect may occur. Adverse effects correspond to those of the components.
Sulfasalazine (salazosulfapyridine)
Although initially developed as an antirheumatic agent, sulfasalazine (salazosulfapyridine) is used mainly in the treatment of inflammatory bowel disease (ulcerative colitis and terminal ileitis or Crohn’s disease).
Gut bacteria split this compound into the sulfonamide sulfapyridine and mesalamine (5-aminosalicylic acid). The latter is probably the anti-inflammatory agent (inhibition of synthesis of chemotactic signals for granulocytes, and of H2O2 formation in mucosa), but must be present on the gut mucosa in high concentrations.
Coupling to the sulfonamide prevents premature absorption in upper small bowel segments. The cleaved-off sulfonamide can be absorbed and may produce typical adverse effects (see above).
Dapsone
Dapsone has several therapeutic uses: besides treatment of leprosy, it is used for prevention/prophylaxis of malaria, toxoplasmosis, and actinomycosis.