This post is an answer to the Case – Patient with a History of IV Drug Abuse
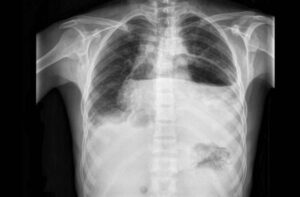
The patient’s admission chest radiograph revealed bilateral widespread nodular infiltrates. The size of these nodules varied and some of them were cavitated.
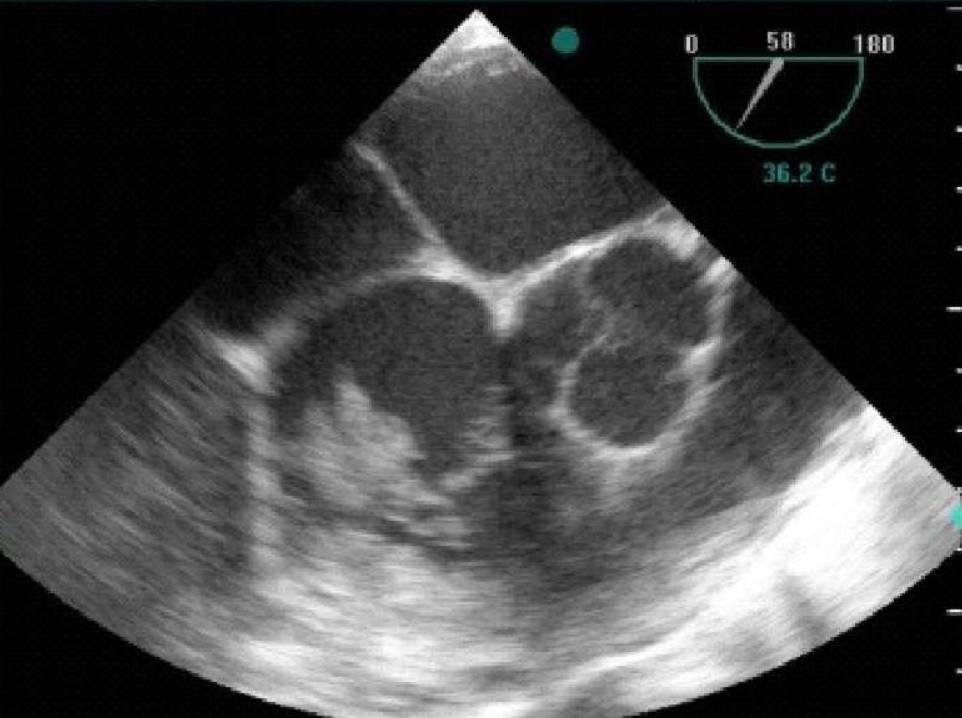
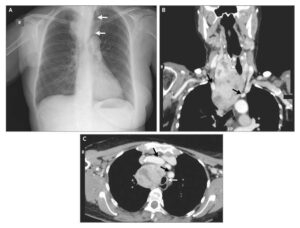
The patient subsequently underwent heart echocardiography (both transthoracic and transoesophageal) where vegetations were found in the tricuspid valve (Fig. 1). Otherwise, the heart function was normal.
The patient was then referred for chest Computed Tomography which confirmed the presence of multiple and widespread irregular pulmonary nodules in both lungs. Some of these nodules were cavitated. The “feeding vessel sign” was also noted in some of the nodules which were directly connected with a vessel. There was also a small left pleural effusion (Fig. 2).
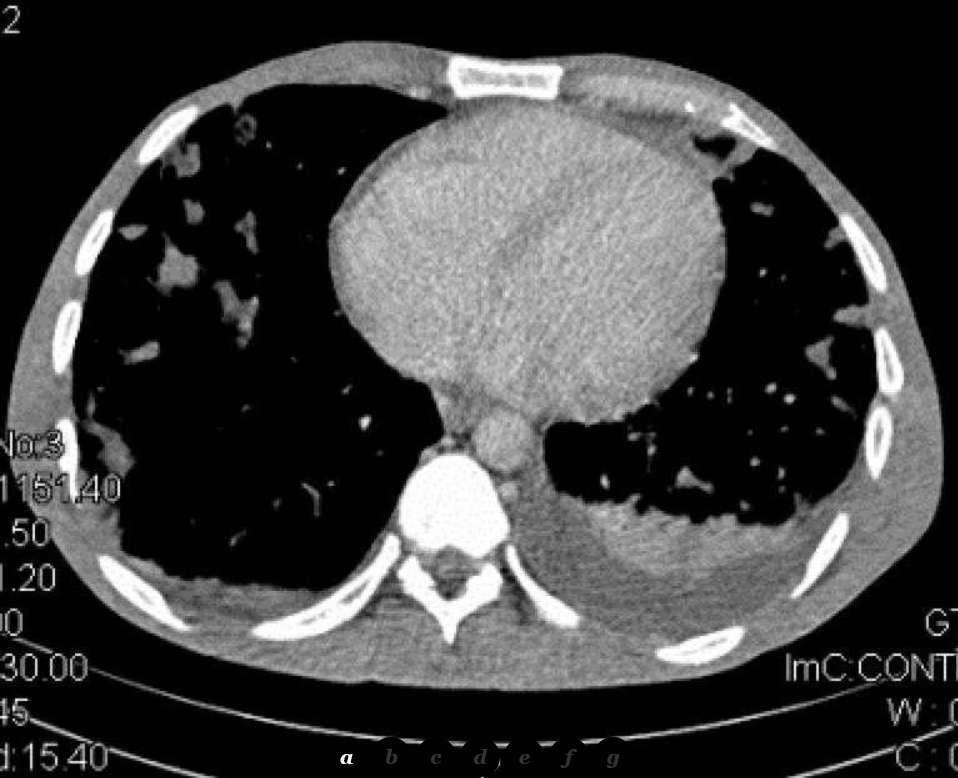
Multi-Planar Reconstruction further helped in interpreting the radiographic findings and in evaluating the extent of the disease.
Differential Diagnosis
- Septic pulmonary embolism caused by tricuspid valve infective endocarditis.
- Septic pulmonary embolism
- Thromboembolism
- Pneumonia
- Metastases
- Foreign body embolus or other type of embolism
- Wegener’s granulomatosis
Discussion about Pulmonary septic embolism (PSE)
Pulmonary septic embolism (PSE) describes the blocking of a pulmonary artery by an infected thrombus which originates from endocarditis, infected catheters, abscesses, pulmonary or urinary infections.
Risk factors include IV drug abuse, alcoholism, skin infection and immunodeficiency [1, 2, 3]. The commonest pathogen of endocarditis is Staphylococcus aureus [4]. Patients with PSE usually present with fever, dyspnoea, pleuritic chest pain and cough [5].
The findings in plain radiographies are usually nonspecific and include multiple bilateral, poorly marginated infiltrates located in the lower lobes which may be cavitated. There may also be signs of pleural effusion [3].
The typical CT image of PSE comprises multiple round densities with or without cavitation which are located diffusely in both lungs but with a lower lobe predilection. There may also be a pericardial or pleural effusion and air bronchogram within pulmonary nodules. Septic foci are less distinctly demarcated than metastases and may have a wedge-like shape with their base on the pleura [1, 2].
With the use of an IV contrast medium, the densities are enhanced in their periphery or in a rimlike pattern. When the septic infiltrates subside, follow-up CT examinations may reveal subpleural linear strands [3]. Empyema is a common complication of PSE which can be discovered by CT [6]. The “feeding vessel sign” characterizes PSE and describes the connection of the density with a distinct pulmonary vessel leading into its centre and proves the haematogenous origin of the lesion. It is easily identified on CT when the feeding vessel lies in the same plane with the nodule [2, 3]. Albeit its high prevalence, the feeding vessel sign may not be true, as recent studies showed that MDCT mutliplanar images prove some feeding vessels to course around the nodules or to represent pulmonary veins. The same sign may also be caused by metastases [7].
Septic emboli can be differentiated from Wegener’s granulomatosis as they are usually smaller, peripheral and may be combined with reactive lymphadenopathy [8]. Vascular filling defects along with the patient’s history (deep vein thrombosis instead of endocarditis) help discriminate septic and thrombotic embolism. Other types of pulmonary embolism like fat or foreign body should be also included in the differential diagnosis [6].
Regarding endocarditis, heart ultrasonography is the primary imaging modality, as it visualises vegetations inside the heart and establishes the diagnosis [4].
Treatment includes antibiotics, removal of infected devices and in rare cases surgery [5].
References
- Dahnert W. (2007) Radiology Review Manual. Philadelphia: LippincotWilliams&Wilkins
- Prokop M, Galanski M, Van der MolenAJ, Schaefer-Prokop CM. (2003) Spiral and Multislice Computed Tomography of the Body. Stuttgart: Thieme
- Huang RM, Naidich DP, Lubat E, Schinella R, Garay SM, McCauley DI. (1989) Septic pulmonary emboli: CT-radiographic correlation. AJR Am J Roentgenol 153(1):41-5. (PMID: 2735296)
- Fellah L, Waignein F, Wittebole X, Coche E. (2007) Combined assessment of tricuspid valve endocarditis and pulmonary septic embolism with ECG-gated 40-MDCT of the whole chest. AJR Am J Roentgenol 189(4):W228-30. (PMID: 17885037)
- Cook RJ, Ashton RW, Aughenbaugh GL, RyuJH. (2005) Septic pulmonary embolism: presenting features and clinical course of 14 patients. Chest 128(1):162-6. (PMID: 16002930)
- Han D, Lee KS, Franquet T, Müller NL, Kim TS, Kim H et al. (2003) Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics 23(6):1521-39. (PMID: 14615562)
- Dodd JD, Souza CA, Müller NL. (2006) High-resolution MDCT of pulmonary septic embolism: evaluation of the feeding vessel sign. AJR Am J Roentgenol 187(3):623-9. (PMID: 16928922)
- Martinez F, Chung JH, Digumarthy SR, Kanne JP, Abbott GF, Shepard JA et al. (2012) Common and uncommon manifestations of Wegener granulomatosis at chest CT: radiologic-pathologic correlation. Radiographics 32(1):51-69. (PMID: 22236893)