Table of Contents
Oral anticoagulant medications are commonly prescribed. Whether it is a traditional vitamin K antagonist (VKA) or a novel oral anticoagulant (NOAC) medication, all carry the risk of serious hemorrhage.
Traditional Vitamin K Antagonists
VKAs inhibit vitamin K epoxide reductase, which prevents the formation of vitamin K–dependent coagulation factors II, VII, IX, and X. The most ubiquitous test to assess anticoagulation in patients on VKAs are prothrombin time (PT) and international normalized ratio (INR).
Vitamin K – dependent factor levels must decrease to 20% to 30% of normal values before changes in INR are consistently observed. The most common VKA is warfarin.
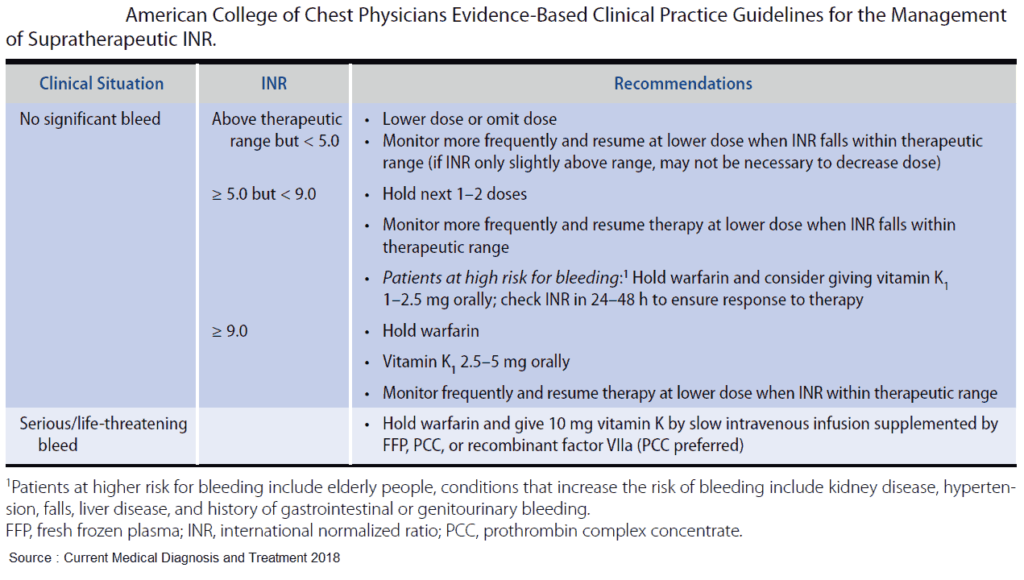
For the nonbleeding patient with an INR > 9, vitamin K should be administered. Available routes of administration include oral, subcutaneous, intramuscular, and intravenous (IV). Though IV administration corrects the INR slightly faster than does the oral route, differences in reversal time are rarely clinically significant. Due to erratic efficacy and risk of intramuscular hematoma formation, subcutaneous and intramuscular routes are not recommended.
For the bleeding patient with any elevated INR, fresh frozen plasma (FFP) should be rapidly administered. FFP directly replaces all vitamin K– dependent factors in a dose-dependent fashion. A dose of 10 to 15 mL/kg is recommended to replace 25% of factor levels.
Prothrombin complex concentrate (PCC) is a concentrated form of vitamin K–dependent factors and is available as 3- or 4-factor products. The 3-factor products contain II, IX, and X, whereas 4-factor PCCs contain II, VII, IX, and X. PCCs deliver a large quantity of factors in a low volume and have fewer side effects when compared with FFP. Current recommended doses for PCCs range between 25 and 50 U/kg. For 3-factor products, some have recommended the addition of activated factor VII to compensate for the insufficient quantities of factor VII.
Currently, the American College of Chest Physicians recommends 4- factor PCCs over FFP in the treatment of life-threatening hemorrhage due to VKAs. To date, trials comparing the efficacy of PCCs to FFP have shown significantly faster correction of INR with PCCs, but have failed to find a significant difference in mortality.
Novel Oral Anticoagulant (NOAC) Medications
NOACs have recently become an alternative to VKAs. Current products include direct thrombin inhibitors (i.e., dabigatran) and factor Xa inhibitors (i.e., rivaroxaban, apixaban, edoxaban). These products do not deplete factors like VKAs; rather, they cause active inhibition. In pharmaceutical sponsored trials, NOACs were shown to be noninferior to warfarin in both efficacy and risk profile.
Traditional anticoagulation tests (i.e., PT, PTT, INR) are inaccurate, insensitive, and unable to quantify the degree of anticoagulation in patients taking an NOAC. Instead, a thrombin time or ecarin clotting time is recommended to evaluate direct thrombin inhibition, whereas anti–factor Xa levels should be obtained when factor Xa inhibition is suspected. Most NOACs have short half-lives, with anticoagulative effects resolving within 24 hours of the last dose.
Current guidelines recommend the use of 4-factor PCCs, activated factor VII, or factor VIII inhibitor bypassing activity to treat hemorrhage from NOACs. Recent trials have demonstrated variable success when using these products to reverse the effects of NOACs.
In contrast to Xa inhibitors, dabigatran can be partially cleared with hemodialysis. Volunteer studies and case reports have demonstrated that up to 68% of circulating dabigatran can be dialyzed in a single 2- to 4-hour session. The clinical presentation and the degree of blood loss will determine the viability of dialysis as a treatment option.
The Food and Drug Administration recently approved reversal agents for both dabigatran and the Xa inhibitors.
Idarucizumab is a monoclonal antibody fragment that binds dabigatran in the serum and neutralizes its effect.
Andexanet is a compound that binds to the site of anti-Xa inhibitors, thereby preventing further inhibition of Xa.
To date, trials evaluating these products are industry sponsored and require further independent study. Nonetheless, their use should be considered in the patient with lifethreatening hemorrhage due to an NOAC medication.
Key Points
- Vitamin K is not recommended in patients on warfarin with no active bleeding and an INR < 9.
- The initial dose of FFP for the hemorrhaging patient on warfarin is 10 to 15 mL/kg.
- 4-Factor PCCs will rapidly correct the INR but have not been shown to improve mortality when compared to FFP.
- Dabigatran can potentially be dialyzed in the event of life-threatening hemorrhage or overdose.
- Consider idarucizumab for emergent reversal of hemorrhage due to dabigatran.
Read Also about Reversal of Anticoagulant Therapy
Suggested Readings
- Chen BC, Sheth NR, Dadzie KA, et al. Hemodialysis for the treatment of pulmonary hemorrhage from dabigatran overdose. Am J Kidney Dis. 2013;62(3):591–594. (https://pubmed.ncbi.nlm.nih.gov/23597859/)
- Dezee KJ, Shimeall WT, Douglas KM, et al. Treatment of excessive anticoagulation with phytonadione (vitamin K): A meta-analysis. Arch Intern Med. 2006;166(4):391–397 (https://pubmed.ncbi.nlm.nih.gov/16505257/)
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(Suppl 1):S141–S145. (https://pubmed.ncbi.nlm.nih.gov/22473649/)
- Pollack CV, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015; 373:511–520. (E-Pub ahead of print.) (https://www.nejm.org/doi/full/10.1056/nejmoa1502000)
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: A randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–1243. (https://pubmed.ncbi.nlm.nih.gov/23935011/)