Table of Contents
Anemia denotes a reduction in red blood cell count, hemoglobin content, or both. Oxygen (O2) transport capacity is decreased.
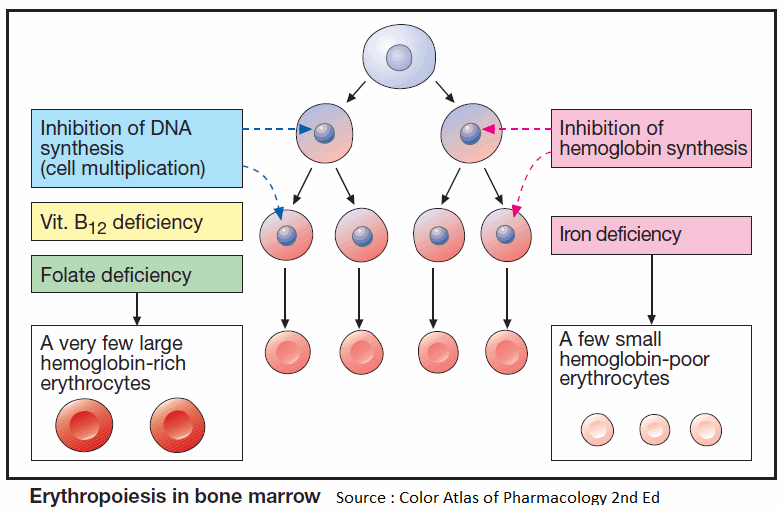
Erythropoiesis
Blood corpuscles develop from stem cells through several cell divisions. Hemoglobin is then synthesized and the cell nucleus is extruded. Erythropoiesis is stimulated by the hormone erythropoietin (a glycoprotein), which is released from the kidneys when renal O2 tension declines.
Given an adequate production of erythropoietin, a disturbance of erythropoiesis is due to two principal causes:
- Cell multiplication is inhibited because DNA synthesis is insufficient. This occurs in deficiencies of vitamin B12 or folic acid (macrocytic hyperchromic anemia).
- Hemoglobin synthesis is impaired. This situation arises in iron deficiency, since Fe2+ is a constituent of hemoglobin (microcytic hypochromic anemia).
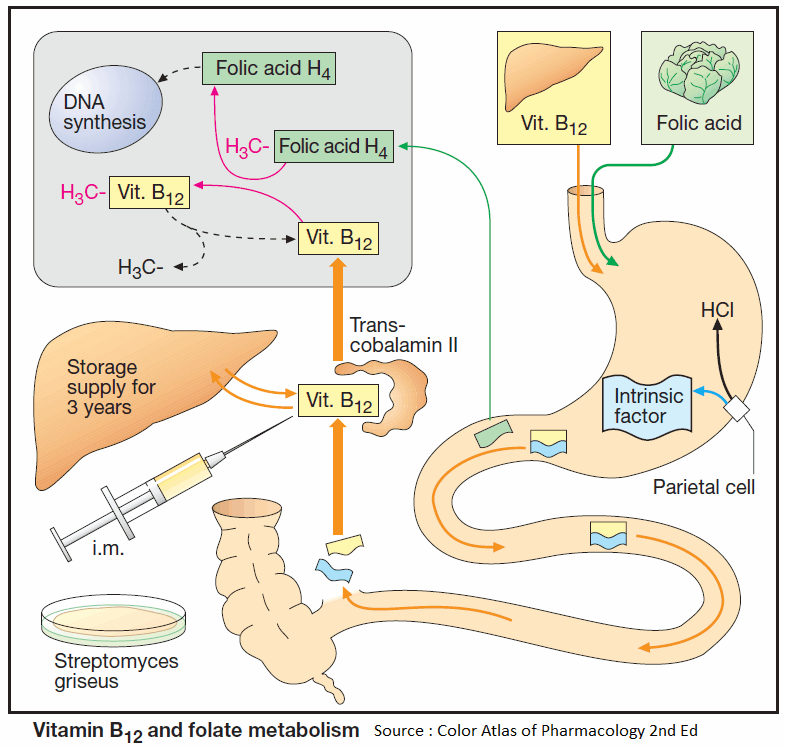
Vitamin B12 (cyanocobalamin)
Vitamin B12 (cyanocobalamin) is produced by bacteria; B12 generated in the colon, however, is unavailable for absorption (see below). Liver, meat, fish, and milk products are rich sources of the vitamin. The minimal requirement is about 1 μg/d.
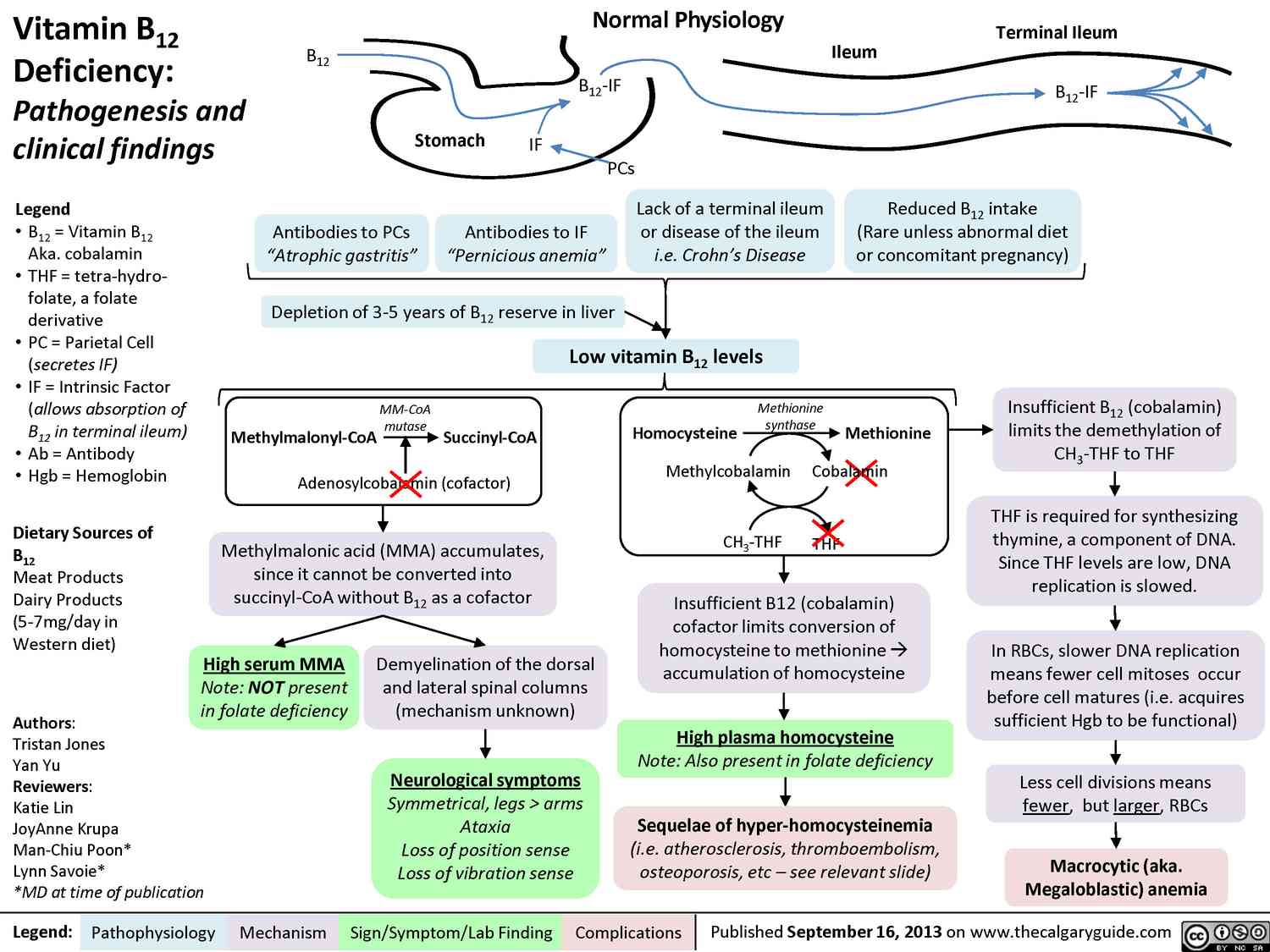
Enteral absorption of vitamin B12 requires so-called “intrinsic factor” from parietal cells of the stomach. The complex formed with this glycoprotein undergoes endocytosis in the ileum. Bound to its transport protein, transcobalamin, vitamin B12 is destined for storage in the liver or uptake into tissues.
A frequent cause of vitamin B12 deficiency is atrophic gastritis leading to a lack of intrinsic factor. Besides megaloblastic anemia, damage to mucosal linings and degeneration of myelin sheaths with neurological sequelae will occur (pernicious anemia).
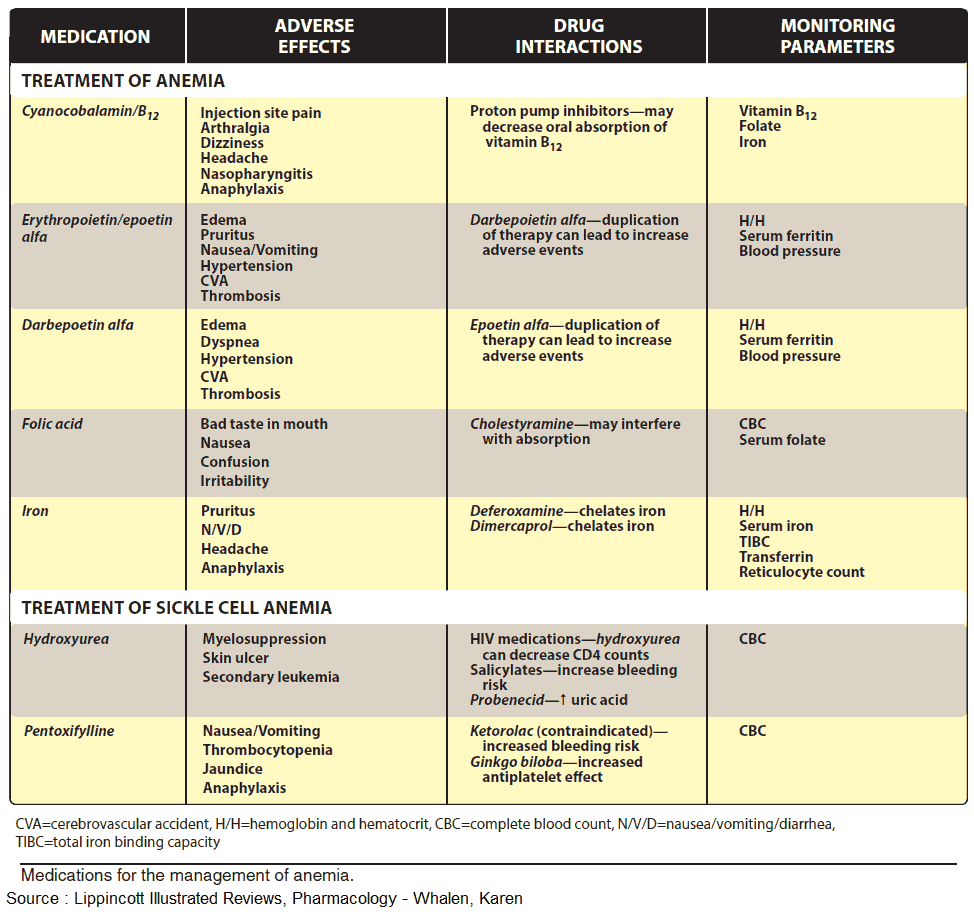
Optimal therapy consists in parenteral administration of cyanocobalamin or hydroxycobalamin (Vitamin B12a; exchange of -CN for -OH group). Adverse effects, in the form of hypersensitivity reactions, are very rare.
Folic Acid
Leafy vegetables and liver are rich in folic acid (FA). The minimal requirement is approx. 50 μg/d. Polyglutamine-FA in food is hydrolyzed to monoglutamine-FA prior to being absorbed. Folic acid is heat labile.
Causes of deficiency include:
- Insufficient intake
- Malabsorption in gastrointestinal diseases
- Increased requirements during pregnancy
- Antiepileptic drugs (phenytoin, primidone, phenobarbital) may decrease FA absorption, presumably by inhibiting the formation of monoglutamine- folic acid
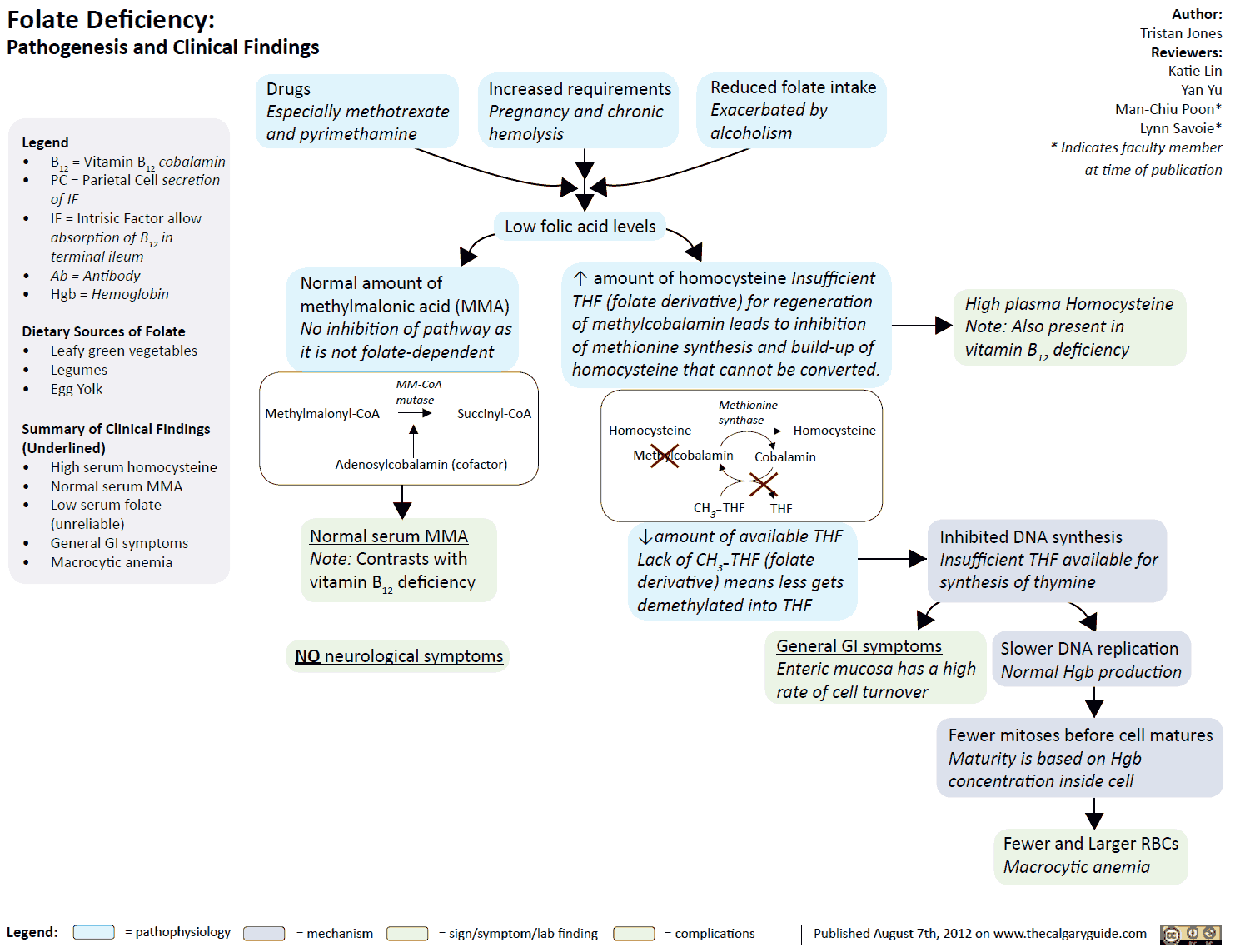
- Inhibition of dihydro-folic acid reductase (e.g., by methotrexate) depresses the formation of the active species, tetrahydro-folic acid
Symptoms of deficiency are:
- megaloblastic anemia
- mucosal damage
Therapy of folic acid deficiency
Therapy consists in oral administration of Folic Acid or folinic acid when deficiency is caused by inhibitors of dihydro—FA—reductase.
Administration of Folic Acid can mask a vitamin B12 deficiency.
Vitamin B12 is required for the conversion of methyltetrahydro- FA to tetrahydro-FA, which is important for DNA synthesis. Inhibition of this reaction due to B12 deficiency can be compensated by increased FA intake.
The anemia is readily corrected; however, nerve degeneration progresses unchecked and its cause is made more difficult to diagnose by the absence of hematological changes. Indiscriminate use of Folic acid-containing multivitamin preparations can, therefore, be harmful.
Iron Compounds
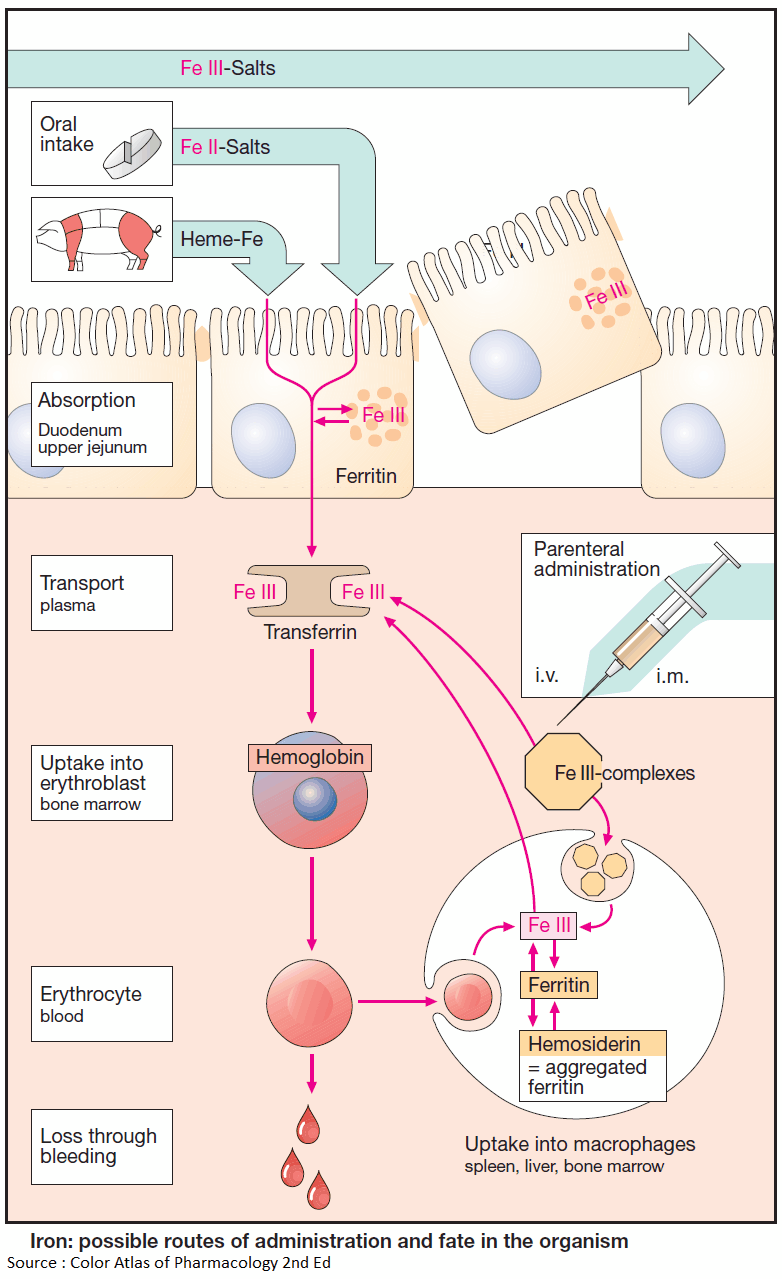
Not all iron ingested in food is equally absorbable. Trivalent Fe3+ is virtually not taken up from the neutral milieu of the small bowel, where the divalent Fe2+ is markedly better absorbed. Uptake is particularly efficient in the form of heme (present in hemo- and myoglobin).
Within the mucosal cells of the gut, iron is oxidized and either deposited as ferritin (see below) or passed on to the transport protein, transferrin, a β1-glycoprotein. The amount absorbed does not exceed that needed to balance losses due to epithelial shedding from skin and mucosae or hemorrhage (so-called “mucosal block”).
In men, this amount is approx. 1 mg/d; in women, it is approx. 2 mg/d (menstrual blood loss), corresponding to about 10% of the dietary intake.
The transferrin-iron complex undergoes endocytotic uptake mainly into erythroblasts to be utilized for hemoglobin synthesis. About 70% of the total body store of iron (~5 g) is contained within erythrocytes. When these are degraded by macrophages of the reticuloendothelial (mononuclear phagocyte) system, iron is liberated from hemoglobin. Fe3+ can be stored as ferritin (= protein apoferritin + Fe3+) or returned to erythropoiesis sites via transferrin.
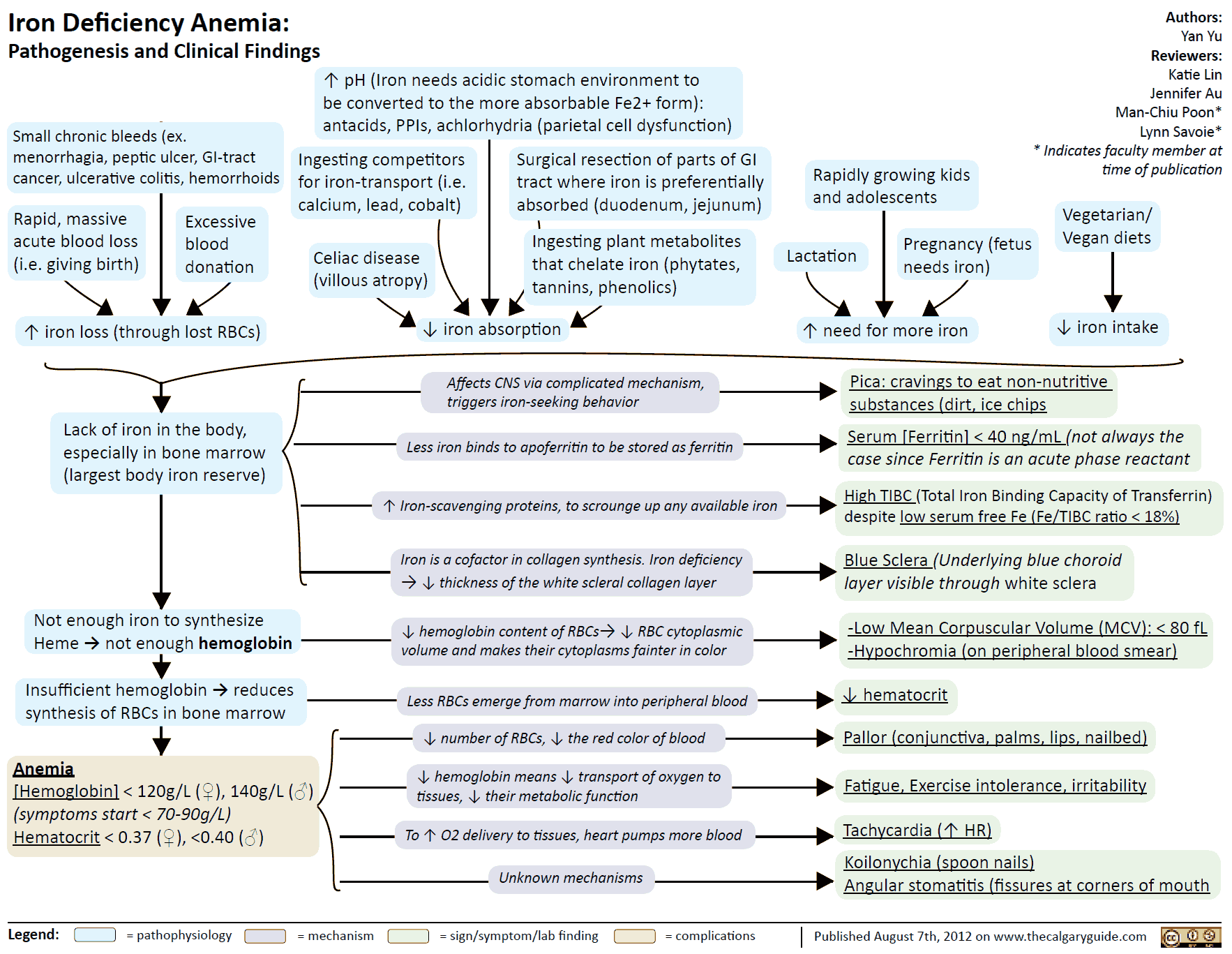
A frequent cause of iron deficiency is chronic blood loss due to gastric/intestinal ulcers or tumors. One liter of blood contains 500 mg of iron. Despite a significant increase in absorption rate (up to 50%), absorption is unable to keep up with losses and the body store of iron falls. Iron deficiency results in impaired synthesis of hemoglobin and anemia.
Treatment of Iron Deficiency
The treatment of choice (after the cause of bleeding has been found and eliminated) consists of the oral administration of Fe2+ compounds, e.g., ferrous sulfate (daily dose 100 mg of iron equivalent to 300 mg of FeSO4, divided into multiple doses). Replenishing of iron stores may take several months.
Oral administration, however, is advantageous in that it is impossible to overload the body with iron through an intact mucosa because of its demand-regulated absorption (mucosal block).
Parenteral administration of Fe3+ salts is indicated only when adequate oral replacement is not possible. There is a risk of overdosage with iron deposition in tissues (hemosiderosis). The binding capacity of transferrin is limited and free Fe3+ is toxic. Therefore, Fe3+ complexes are employed that can donate Fe3+ directly to transferrin or can be phagocytosed by macrophages, enabling iron to be incorporated into ferritin stores.
Adverse effects
The frequent gastrointestinal complaints (epigastric pain, diarrhea, constipation) necessitate intake of iron preparations with or after meals, although absorption is higher from the empty stomach.
Possible adverse effects are, with i.m. injection: persistent pain at the injection site and skin discoloration; with i.v. injection: flushing, hypotension, anaphylactic shock.
Interactions
Antacids inhibit iron absorption. Combination with ascorbic acid (Vitamin C), for protecting Fe2+ from oxidation to Fe3+, is theoretically sound, but practically is not needed.