Table of Contents
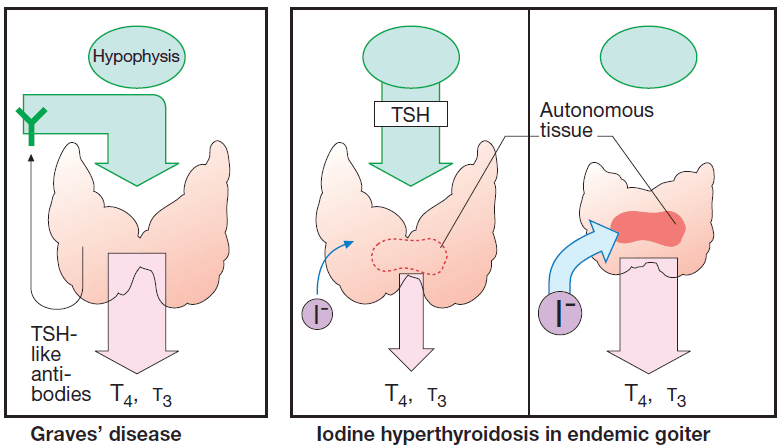
Thyroid overactivity in Graves’ disease results from formation of IgG antibodies that bind to and activate TSH receptors. Consequently, there is overproduction of hormone with cessation of TSH secretion.
Graves’ disease can abate spontaneously after 1–2 y. Therefore, initial therapy consists of reversible suppression of thyroid activity by means of antithyroid drugs.
In other forms of hyperthyroidism, such as hormone – producing (morphologically benign) thyroid adenoma, the preferred therapeutic method is removal of tissue, either by surgery or administration of 131iodine in sufficient dosage. Radioiodine is taken up into thyroid cells and destroys tissue within a sphere of a few millimeters by emitting β-(electron) particles during its radioactive decay.
Read Also : Recognizing and Treating Thyroid Storm
Antithyroid Drugs
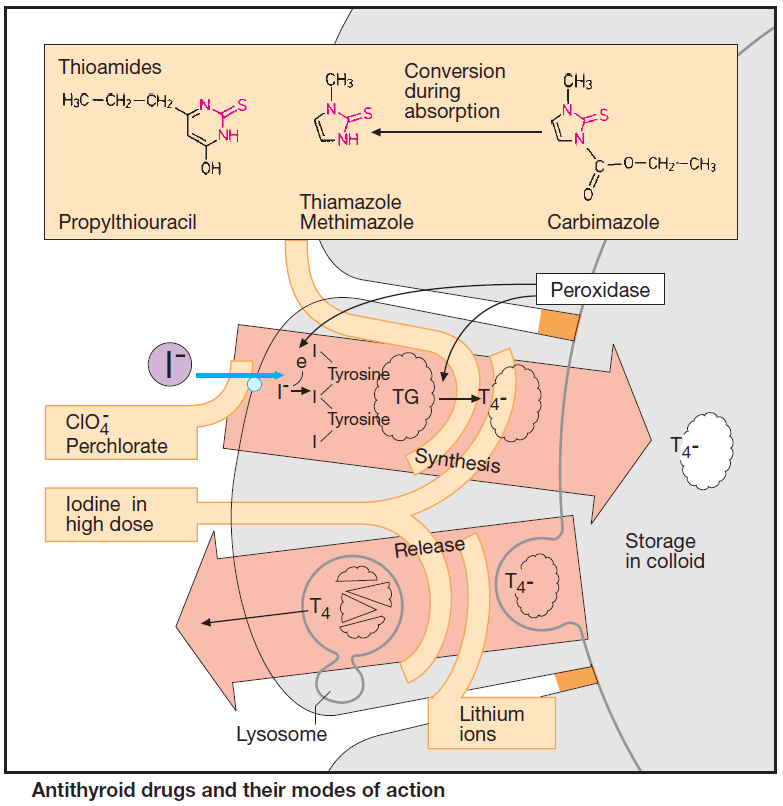
Antithyroid drugs inhibit thyroid function. Release of thyroid hormone is preceded by a chain of events. A membrane transporter actively accumulates iodide in thyroid cells; this is followed by oxidation to iodine, iodination of tyrosine residues in thyroglobulin, conjugation of two diiodotyrosine groups, and formation of T4 and T3 moieties.
These reactions are catalyzed by thyroid peroxidase, which is localized in the apical border of the follicular cell membrane. T4-containing thyroglobulin is stored inside the thyroid follicles in the form of thyrocolloid. Upon endocytotic uptake, colloid undergoes lysosomal enzymatic hydrolysis, enabling thyroid hormone to be released as required.
A “thyrostatic” effect can result from inhibition of synthesis or release. When synthesis is arrested, the antithyroid effect develops after a delay, as stored colloid continues to be utilized.
Antithyroid drugs for long-term therapy
1. Thiourea Derivatives (thioureylenes, thioamides)
Thiourea derivatives (thioureylenes, thioamides) inhibit peroxidase and, hence, hormone synthesis. In order to restore a euthyroid state, two therapeutic principles can be applied in Graves’ disease:
- a) monotherapy with a thioamide with gradual dose reduction as the disease abates;
- b) administration of high doses of a thioamide with concurrent administration of thyroxine to offset diminished hormone synthesis.
Adverse effects of thioamides are rare; however, the possibility of agranulocytosis has to be kept in mind.
2. Perchlorate
Perchlorate, given orally as the sodium salt, inhibits the iodide pump.
Adverse reactions include aplastic anemia.
Compared with thioamides, its therapeutic importance is low but it is used as an adjunct in scintigraphic imaging of bone by means of technetate when accumulation in the thyroid gland has to be blocked.
Short-term thyroid suppression
1. Iodine in high dosage
Iodine in high dosage (>6000 μg/d) exerts a transient “thyrostatic” effect in hyperthyroid, but usually not in euthyroid, individuals. Since release of thyroid hormones is also blocked, the effect develops more rapidly than does that of thioamides.
Clinical applications include:
- Preoperative suppression of thyroid secretion according to Plummer with Lugol’s solution (5% iodine + 10% potassium iodide, 50–100 mg iodine/d for a maximum of 10 d).
- In thyrotoxic crisis, Lugol’s solution is given together with thioamides and β-blockers.
Adverse effects: allergies;
Contraindications: iodine-induced thyrotoxicosis.
2. Lithium ions
Lithium ions inhibit thyroxine release. Lithium salts can be used instead of iodine for rapid thyroid suppression in iodine-induced thyrotoxicosis.